- high-resolution terminology - matching measurements at high-resolution
Cell count and normalization in HRR
Description
The cell count Nce is the number of cells, expressed in the abstract unit [x] (1 Mx = 106 x). The elementary entity cell Uce [x] is the real unit, the 'single individual cell'. A cell count is the multitude or number N of cells, Nce = N·Uce (Gnaiger MitoFit Preprints 2020.4). Normalization of respiratory rate by cell count yields oxygen flow IO2 expressed in units [amol·s-1·x-1] (=10-18 mol·s-1·x-1).
Abbreviation: Nce
Reference: Gnaiger 2020 BEC MitoPathways, Gnaiger MitoFit Preprints 2020.4
Communicated by Gnaiger Erich (2020-09-18) last update 2020-10-24 Contributions by Cardoso Luiza HD, Schmitt Sabine, Sobotka Ondrej, Zdrazilova Lucie
Cell count and cell concentration
- Cells are frequently pipetted into the O2k-chamber. Start with chamber and stopper in volume-calibrated experimental position (C), having siphoned off any aqueous medium from the receptacle of the stopper. During moving off the stopper, the medium in the stopper capillary is drawn into the chamber. Then the experimental chamber volume V containing respiration medium of 0.5 mL or 2.0 mL in the two types of O2k-chambers is automatically increased by the dead volume Vstc of the stopper capillaries, corresponding to 0.04 mL or 0.07 mL medium, respectively. If the volume of the stopper capillary is ignored in the calculation of cell-count concentration, then the final cell-count concentration in the respirometric chamber is underestimated by 7 % and 4 %, respectively. V´ including the volume of the stopper capillary is 7 % and 4 % larger than the calibrated experimental volume of V=0.5 mL or 2.0 mL using the 0.5-mL and 2.0-mL chamber, respectively. The corresponding corrections, however, depend on the technical details of the procedures used when adding cell suspensions into the chamber with stopper off and removed (Figure 1), as summarized in the following sections.
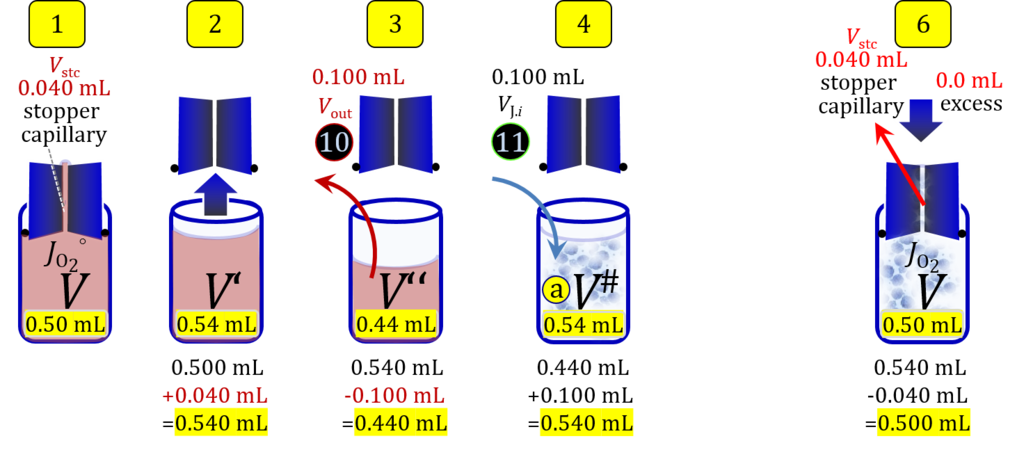
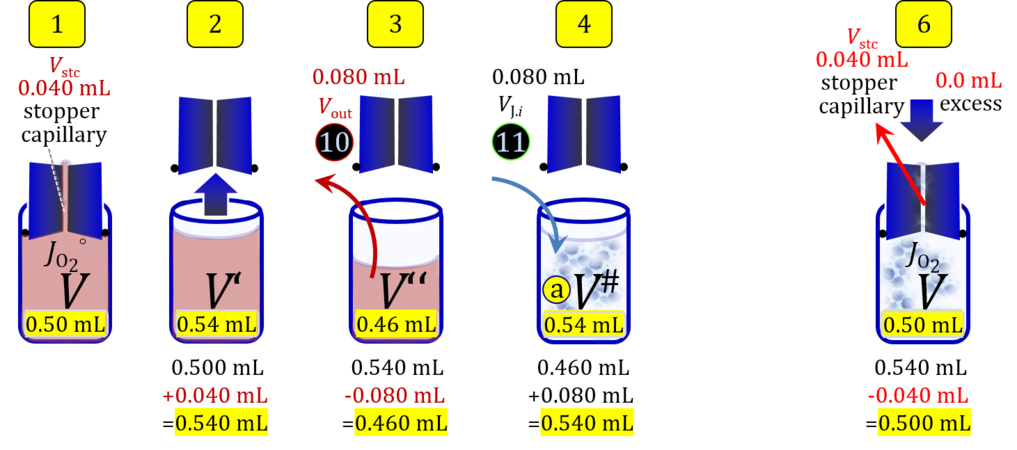
Volume balance in the experimental chamber
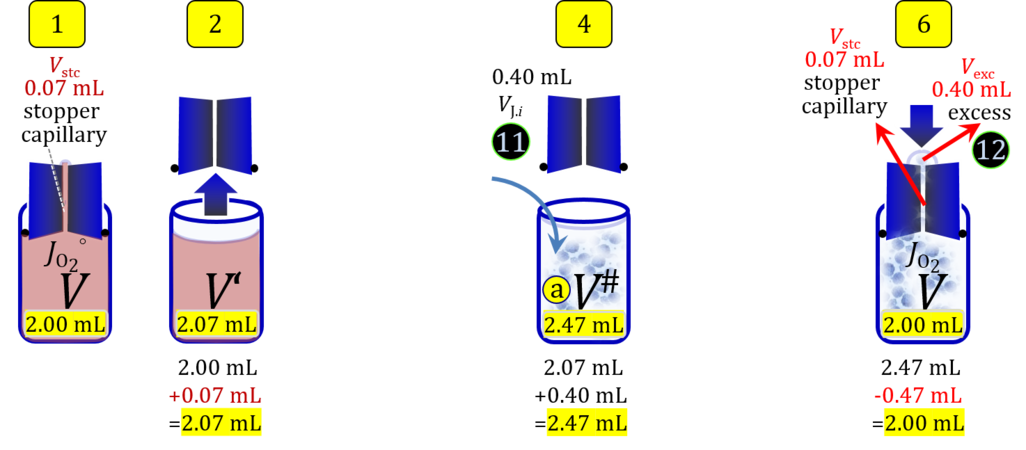
- When a cell suspension is added to the O2k-chamber with the stopper moved off its volume-calibrated position, the volume balance in the O2k-chamber depends on complete or partial volume replacement (CVR or PVR; Figure 2). After the O2k-chamber filled with experimental respiration medium is closed with the stopper in the volume-calibrated position, any excess medium is siphoned off the receptacle of the stopper (1). Then the stopper is gently removed from the chamber (2) and placed into an empty 50-mL Falcon on the Tube Rack (Figure 1). The aqueous medium in the stopper capillary is sucked out of the capillary when moving the stopper up to the edge of the glass chamber, by the negative pressure exerted when moving the stopper upwards. The stopper must not be dried. A volume VJ.i of respiration medium is pipetted off the chamber and discharged (3). The same or larger volume VJ.i of stock cell suspension J is pipetted into the chamber (4). The stirrer may be switched off during this time to avoid foam formation. The stirrer is switched on before taking any subsample from the cell suspension (5). Any drop remaining on to the lower conical surface or up to the lower O-ring is returned to the chamber volume when closing the chamber. Thus the effective dilution by the aqueous content of the stopper capillary is the same, whether the stopper is removed with or without a drop on the lower end of the stopper. When the chamber is closed with the stopper, the experimental volume is V, the stopper capillary is full with experimental medium, if no drops are lost from the removed stopper and no medium is lost by evaporation, and any excess volume of cell suspension Vexc is siphoned off the stopper receptacle (6). If any aqueous medium is lost from the stopper while it is removed, then the capillary would not be re-filled completely, and the dilution would be less. Or you would even see a gas bubble remaining after closing the chamber. Addition of medium to replace the gas bubble would dilute the sample as if the entire drop of medium would have been returned with the stopper.
Cell concentration from cell suspension to experimental chamber
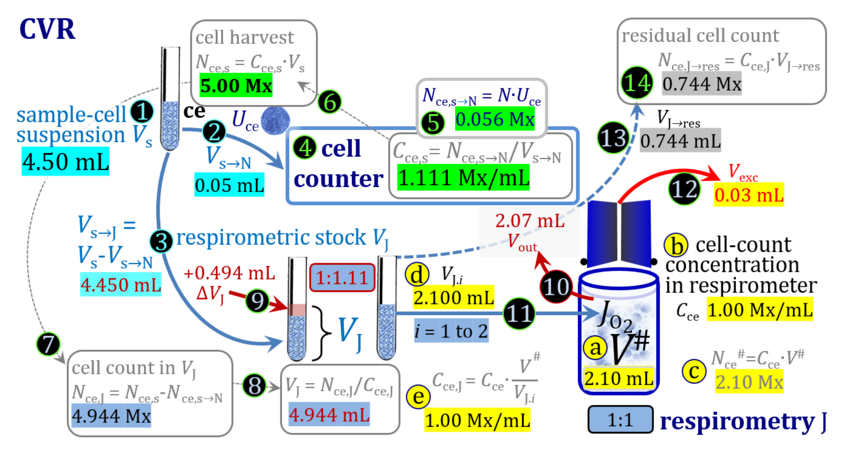
- The harvest of cultured cells typically includes first a centrifugation step to separate the cells from the culture medium, after which the pellet is taken up by adding a defined volume ΔVs of experimental medium to yield a harvested cell suspension with total sample volume Vs. Since the volume of the pellet is not known accurately, the total volume Vs is more accurate when ΔVs is very much larger compared to the volume of the pellet, which in addition leads to a higher dilution of any remaining aqueous medium in the pellet. Subsamples of Vs are used for cell counting (N), additional assays, and respirometry (J), either directly (concentrations in J and s are equal) or after further dilution from s to stock J. Subsamples are transferred from the stock cell suspension J into the O2k-chamber in a variety of ways, selection of which depends on the optimum treatment of the specific cell suspension and practical considerations on the accuracy of titrations.
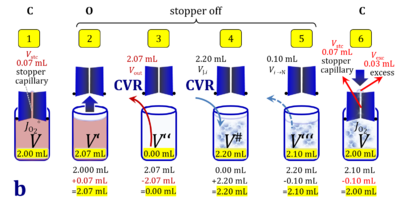
1. Complete volume replacement CVR
- The stock cell-count concentration Cce,J is equal to the initial cell-count concentration Cce in the experimental chamber (Figure 3a (b) and (e)). The stopper is removed from the O2k-chamber and placed into an empty 50-mL Falcon on the Tube Rack (Figure 1). Aqueous medium is siphoned off from the chamber and discharged. No drops remain in the chamber (V´´ = 0 mL; Figure 3b (3)). When a surplus of cell suspension is available, a small volume (0.1 mL) may be used for an initial wash. A volume VJ.i = V# (equal or higher than the experimental chamber volume V plus volume of stopper capillary) is added into the empty chamber. The stirrer may be left on, but is switched off if this helps to avoid foam formation. The stirrer must be switched on before taking subsamples from the chamber, and before closing the chamber with the stopper, which must have a dry conical plate and dry capillary, but wet O-rings. In the example shown in Figure 3, the volume V# is 2.10 mL, leaving a small excess volume Vexc = 0.015 mL as a safety margin for avoiding any bubbles and completely filling the stopper capillary when closing the chamber. The small excess volume of suspension is siphoned off from the receptacle. The cell-count concentration Cce calculated from the initially determined Cce,s (4) can be compared with the finally determined cell-count concentration Cce.i (14) for quality control of cell counting and dilution. Experimental evidence indicates, that the accuracy and reproducibility of IO2/ce in high-resolution respirometry is limited by the accuracy and reproducibility of cell counting.
- » Download Excel template: File:O2k-2 mL Cell count.xlsx
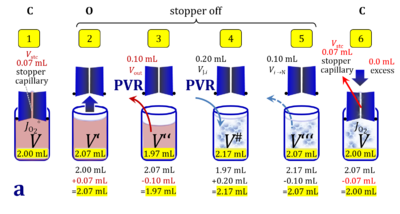
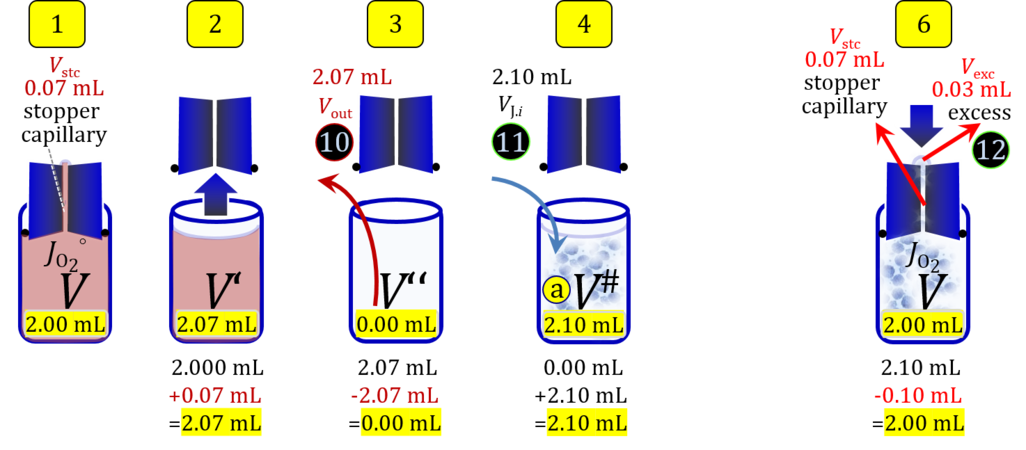
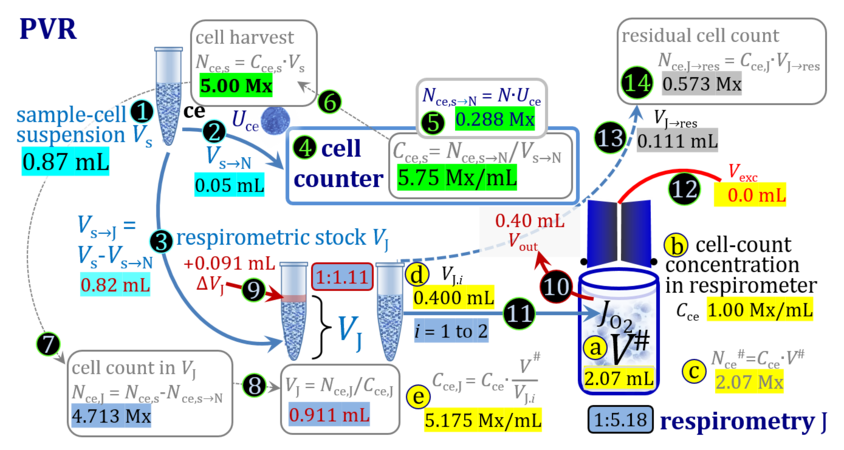
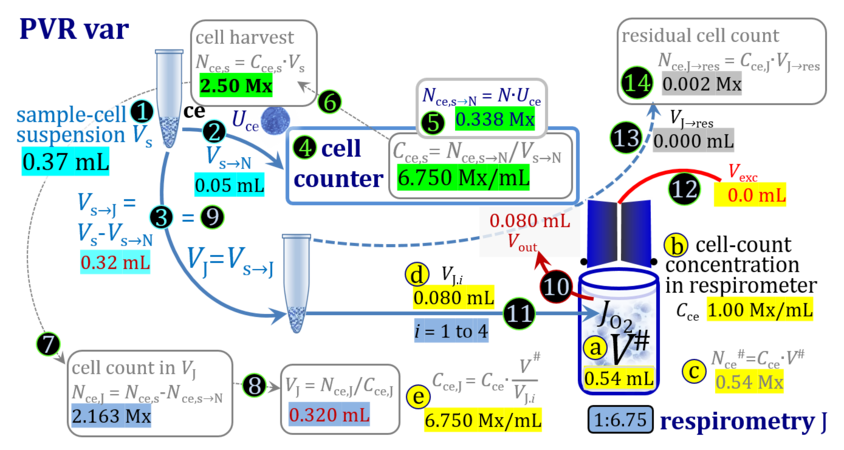
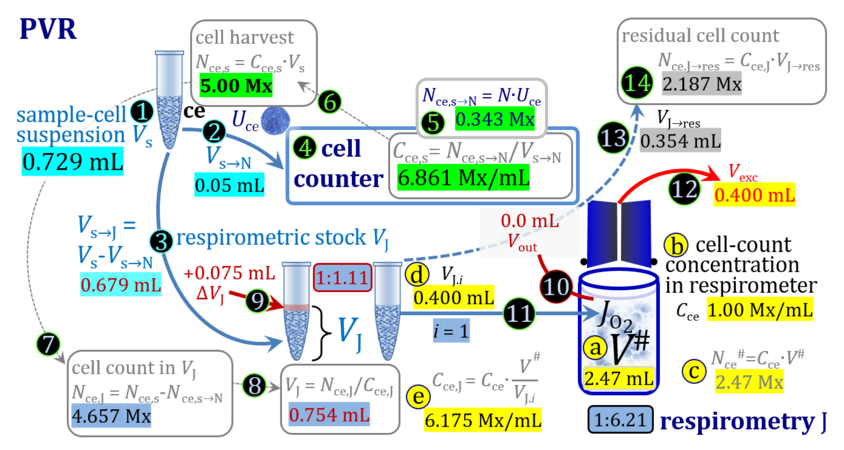
2. Partial volume replacement PVR with Vout
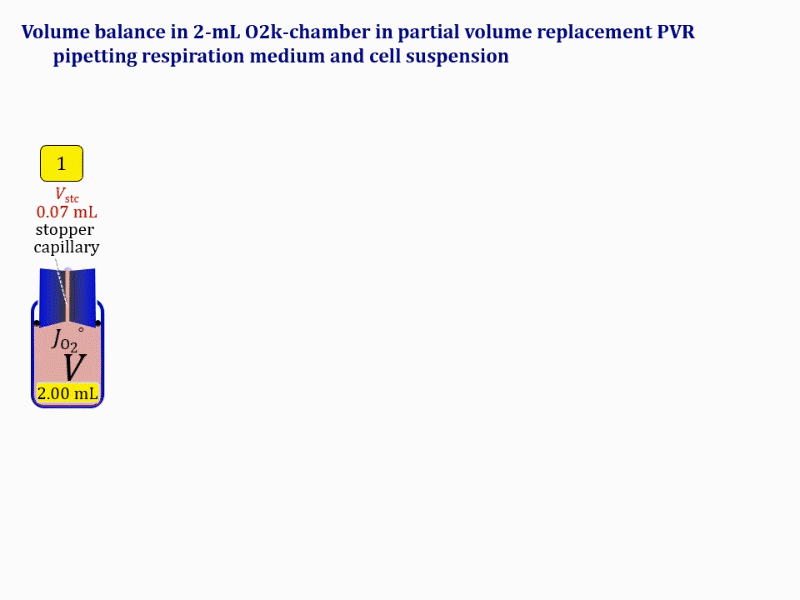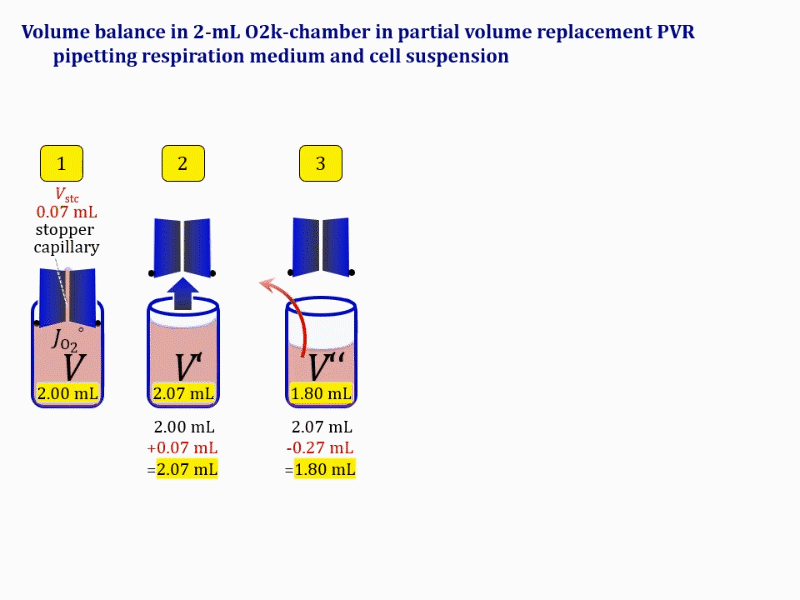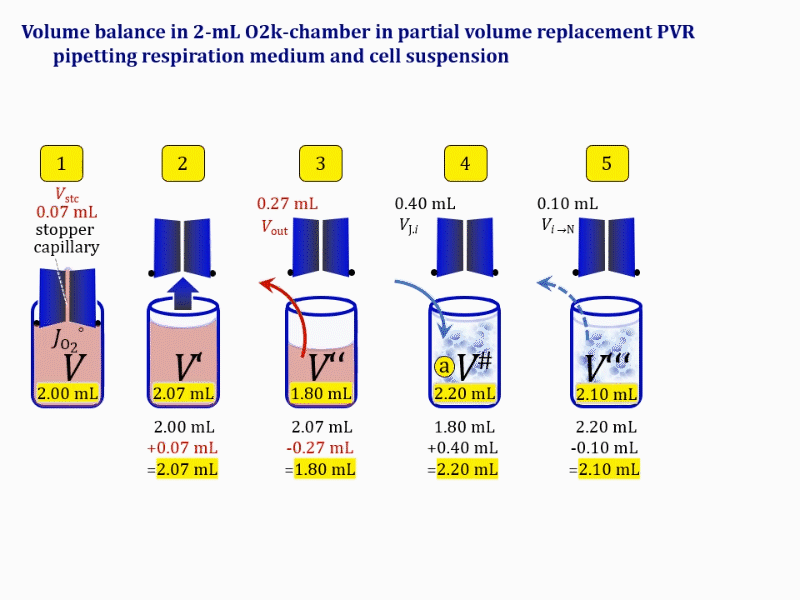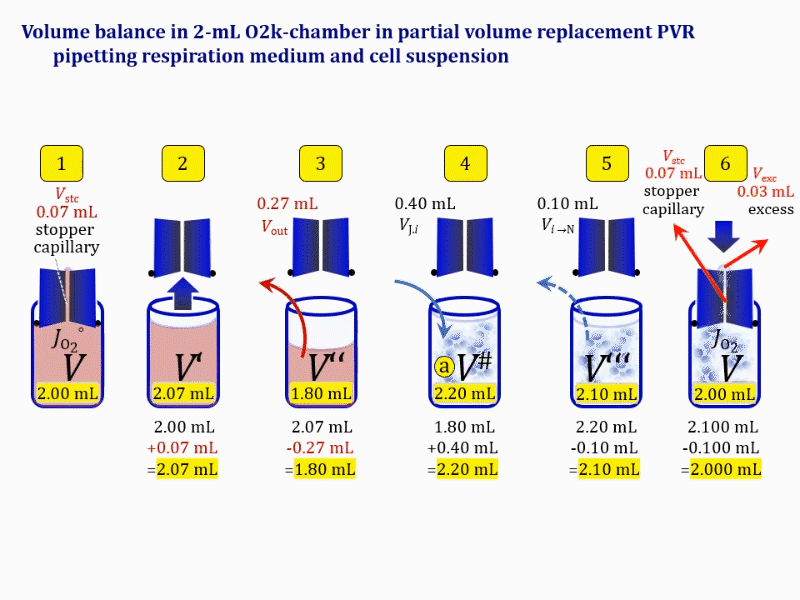
- Figure 4a and 5a show the work flow from cell harvest to filling the respirometric chamber, whereas Figure 4b and 5b zoom into the details of the volume balance in the 2.0-mL and 0.5-mL O2k-chamber, respectively, from moving the stopper off and back onto its volume-calibrated position in the chamber.
Figure 4a. Pipetting a concentrated cell suspension VJ.i into the 2.0-mL O2k-chamber with stopper removed off its volume-calibrated position, after removal a volume of medium from the chamber. The volume V# is obtained after addition of cell suspension and is decisive for calculation of the dilution and cell-count concentration in the chamber. Suspensin of the cell pellet in a volume ΔVs of 0.863 mL in a strategy expecting a total cell count Nce,s* of 4.5 Mx as the minimum, and determined Nce,s of 5.0 Mx.
Figure 4b. Volume balance in the 2-mL O2k-chamber pipetting respiration medium and cell suspension. (1) V: closed chamber with respiration medium, measurement of instrumental O2 background flux JO2°. (2) V´: when the stopper is removed off, the volume Vstc in the stopper capillary is drawn into the chamber. (3) V´´: the volume of respiration medium is reduced by Vout, avoiding an excess volume leading to unnecessary loss of cells. (4) V#: total volume in chamber with stopper off, after addition of cell suspension J. (5) V´´´: volume in chamber with stopper off, after subsampling cell suspension from chamber (omitted). (6) V: experimental chamber volume after replacing the stopper in volume-calibrated position, pushing cell suspension into and through the stopper capillary.
- » Download Excel template: File:O2k-2 mL Cell count.xlsx
- » Download Excel template: File:O2k-0.5 mL Cell count.xlsx
3. Partial volume replacement PVR with variable titration volume
- » Download Excel template: File:O2k-0.5 mL Cell count.xlsx
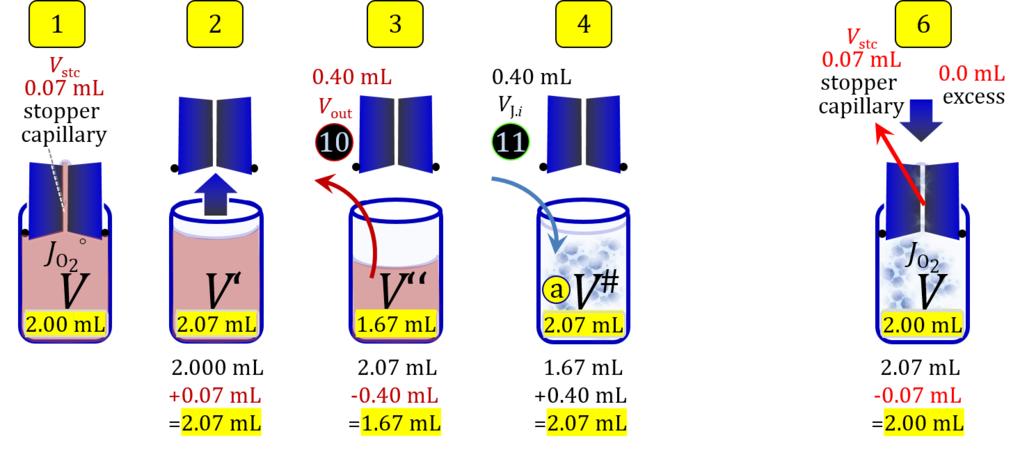
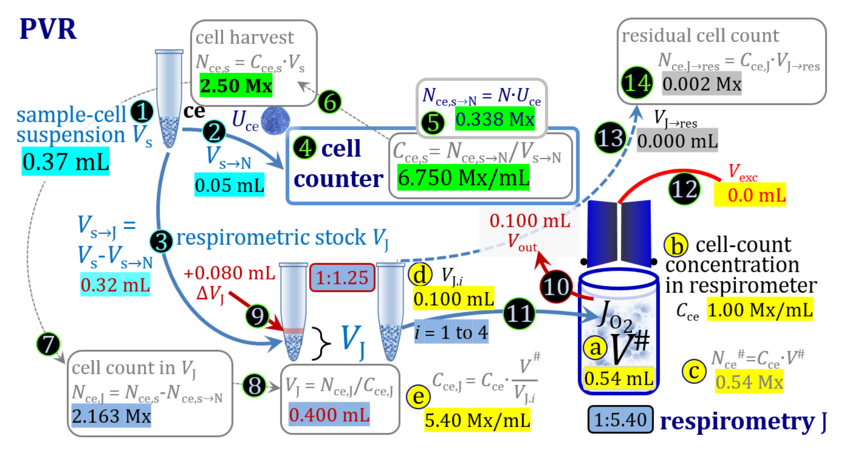
4. Partial volume replacement PVR without Vout
- Figure 7a shows the work flow from cell harvest to filling the respirometric chamber, whereas Figure 7b zooms into the details of the volume balance in the 2.0-mL O2k-chamber from opening to closing the chamber. After the O2k-chamber filled with experimental respiration medium is closed with the stopper in the volume-calibrated position, any excess medium is siphoned off the receptacle of the stopper. Then the stopper is gently removed from the chamber and placed into an empty 50-mL Falcon on the Tube Rack (Figure 1). A volume VJ.i of concentrated stock cell suspension VJ is pipetted into the open chamber. Then the total volume is V# = V+VStopper capillary+VJ.i. A sufficiently large volume of cell suspension is available for taking subsample(s) from the chamber. When the chamber is closed with the stopper, the experimental volume is V, and the stopper capillary is full with experimental medium. An excess volume is siphoned off the receptacle of the stopper. In this way more cells are lost compared to the previous procedure, but an excess volume is available that may be helpful in case of having to remove any trapped gas bubble from the chamber.
Figure 7a. Pipetting a concentrated cell suspension VJ.i into the opened O2k-chamber. Before closing, the volume is V# = V + VStopper capillary + VJ.i, and the excess volume Vexc is extruded through the stopper. Suspension of the cell pellet in a volume ΔVs of 0.724 mL in a strategy expecting a total cell count Nce,s* of 4.5 Mx as the minimum, the determined Nce,s is 5 Mx.
- » Download Excel template: File:O2k-2 mL Cell count.xlsx
5. Titration into closed chamber V
- After the O2k-chamber filled with experimental respiration medium is closed with the stopper in the volume-calibrated position, a volume VJ.i of concentrated stock cell suspension VJ is titrated into the chamber, identical to adding a suspension of isolated mitochondria. This approach is well tested for yeast cells, but is not recommended for mammalian cells which may loose viability due to the high shear stress in a Hamilton syringe. When the titration is performed fast into the O2k-chamber, a volume VJ.i of pure medium is extruded through the stopper, before mixing of cells into the chamber volume, such that no cells are lost from the experimental chamber into the stopper capillary. The dilution of the cell stock concentration in the experimental chamber is then calculated with reference to the chamber volume V. Slow titrations, however, entail an escape of cells into the stopper capillary leading to an inaccurately calculated cell-count concentration Cce. During subsequent titrations in a SUIT protocol, the cell suspension is diluted and excess cell suspension filles the volume of the stopper capillary. When opening the chamber for reoxygenation or decreasing the oxygen level with a gas phase, the cell suspension is pulled back into the open chamber. However, if the chamber is opened without prior SUIT steps, the medium in the stopper capillary does not contain cells and dilutes the cell-number concentration in the chamber.
Normalization of respiratory rate by cell count
- Oxygen flow IO2 [amol·s-1·x-1], normalized for the cell count Nce [x], is as inaccurate and irreproducible as the quantification of cell count. In technical respirometric repeats with the same volume of cells sampled from the same stock, reproducibility of volume-specific oxygen flux JO2 [pmol·s-1·mL-1] depends on the homogeneity of the cell stock and reproducibility of titration of the cell suspension into the experimental chamber. If cell counts are taken for subsamples obtained from each experimental chamber separately, the variability of IO2 and JO2 can be compared to assess the reproducibility of the respirometric measurement versus cell counting.
- A population of living cells includes a majority of viable cells vce, but is characterized by a small fraction of dead cells dce. As a rule, the cell viability index VIce should be equal or higher than 0.95 in a healthy reference cell population. Normalization for the cell count of the total of living cells Nce underestimates mitochondrial function in 'intact' cells, if dead cells have lost all mitochondrial respiratory capacity. On the other hand, normalization for the cell count of only the viable fraction of living cells, Nvce = VIce·Nce, overestimates mitochondrial respiratory function, if mitochondria of dead cells contribute to some extent to oxygen flux. This source of uncertainty of normalization increases with decreasing cell viability index (Gnaiger 1997 Transplant Proc, Steinlechner-Maran 1997 Transplantation, Gnaiger 2000 Transpl Int, Stadlmann 2002 Transplantation, Stadlmann 2006 Cell Biochem Biophys).
Canonical considerations of experimental chamber volume
Canonical Carol Canon O
- The experimental chamber has an operation volume V [mL] in its assembled configuration where the stopper is placed in the volume-calibrated position (Figure 2, step 1: C). The SI unit for volume is cubic meter [m3]. In solution chemistry it is practical to use the unit liter [L] (1 L = 1 dm3; 1 m3 = 1000 dm3 = 1000 L; 1 cm3 = 1 mL).
Instrumental and experimental chamber
- The instrumental O2k-Chamber — a product item in the catalogue — is made of Duran® glass with a mass mglass of 24.3 g and 20.1 g for the 0.5-mL and 2-mL chamber, respectively (Figure 8). At a density ρDuran of 2.23 g·mL-1 at 25 °C and a very low coefficient of thermal expansion, mass is converted to the glass volume VDuran of 10.9 and 9.0 mL for the 0.5-mL and 2-mL chamber, respectively. VDuran is the volume of the instrumental chamber, never considered in the context of a practical experiment nor related to the nomenclature of the 0.5-mL and 2-mL O2k-chambers. The operation volume or experimental volume V (system in thermodynamics) is distinguished from the instrumental chamber volume VDuran. The experimental volume V is enclosed by the boundary between the experimental contents or sample mixture s and the chamber walls. It is appropriate to say that the chamber walls are everything for the instrumental chamber, and are nothing for the experimental chamber. In the case of the experimental O2k-chambers, the system boundary is not only the boundary between the sample s and the glass wall, but includes the boundary between s and the membrane of the oxygen sensor and the sealing of the butyl rubber gasket (OroboPOS-Seal Tip), the boundary between s and the coating of the stirrer bar (black PEEK or white PVDF), the boundary between s and the PEEK stopper sealed by Viton O-rings, and the boundary between s and the aqueous non-stirred volume Vstc in the stopper capillary.
- When the stopper is taken off the volume-calibrated position (Figure 2, stopper off), then the experiment is effectively interrupted and the defined V does not exist in this state. The experimental chamber exists only during the time when it defines and confines V. Upon moving the stopper off a chamber filled with experimental aqueous solution, the aqueous volume Vstc is extruded from the capillary and mixes with the aqueous volume V, such that an aqueous volume V´ = V+Vstc remains as the contents in the glass chamber (Figure 2, step 2: O). The experimental volume V is restored only after inserting the stopper back into it's volume-calibrated position (Figure 2, step 6: C).
- Even after clarification of these apparently minute details, the definition of V remains potentially ambiguous. To address these ambiguities, the following distinctions have to be made: The assembled chamber with stopper in volume-calibrated position (C) may be (1) 'full' including a sample, (2) 'full' but excluding a sample and containing aqueous medium only (for calibration of the oxygen sensor), or (3) 'empty' without aqueous medium.
Full and empty chambers
- V and Vaq: V is the volume of aqueous medium Vaq or the suspension of medium with sample (cell suspension) in the chamber enclosed within the system boundaries. At a cell-count concentration Cce of 1 Mx·mL-1 suspended in aqueous respiration medium and cells with a wet mass per cell of 0.0014 µg·x-1 (1.4 mg·Mx-1; which may apply to fibroblasts, HUVEC or HEK), the cell-mass concentration Cmce in the chamber is 1.4 mg·mL-1. At a density ρce of 1.06 g of cell mass per mL of cell volume, the 'cell-volume concentration' (the volume fraction) of cells in suspension in the experimental chamber is 0.0013. In other words, the cell volume amounts to only 0.13 % of the experimental volume V. Therefore, chemical properties of the cell suspension can be estimated with good approximation by chemical properties of the experimental medium in which the cells are suspended. This applies, for instance, to the oxygen solubility, which is taken as the oxygen solubility of the medium applied to the cell suspension, when Vaq = 0.9987·V. This argument applies only to suspensions at high dilution. It is inappropriate, however, to apply the same argument to a very concentrated cell suspension of adipocytes, which enclose a large volume of fat with high oxygen solubility. In such cases, V and Vaq have to be clearly distinguished. Practically, V is calibrated with pure water (aqueous medium may be used, but would be merely waste of medium). This leads to the definition V ≝ Vaq.
- V and V°: The assembled chamber with stopper in volume-calibrated position (C) may be 'empty'. An empty chamber does not contain aqueous medium (if the chamber in Figure 2a is closed after step 3). Then the aqueous volume is replaced by a volume of gas, which in the simplest case is air, when 'emptiness' is defined in terms of sample and aqueous medium, and V° = V is defined by the geometry of the experimental chamber. Theoretically, emptiness in V° may be defined as vacuum. This leads to two practical concepts on V: (1) a geometric meaning of the system defined simply by the physical dimensions of the experimental chamber independent of its contents, and (2) a 'substantial' meaning of the system related to the contents with which the chamber is completely filled.
Volume or change of volume
- To extend the canonical considerations of volume on a higher level of system analysis, volumes V are defined either as static quantities of various separate systems and subsystems, or as dynamic changes dV or ΔV of volume related to a system with internal transformations (diV or ΔiV; such as thermal volume expansion in a closed system) and external transfer (deV or ΔeV; such as a positive volume change deVin added to an open system, balanced by a negative volume change deVout removed from an open system simultaneously at steady-state and constant system volume V). A quantity volume can only have a positive value, but different volumes may be added or subtracted (volume balance in Figure 2). In contrast, volume changes may be positive or negative, as defined from the perspective of a given system. diV due to thermal volume expansion relates to classical thermodynamics of the Carnot cycle in closed systems, whereas deV due to the transfer of matter relates to open systems at equilibrium, near equilibrium, or far from equilibrium, which are mainly treated in the thermodynamics of irreversible processes.
Definition of terms, symbols and units
- Table 1. Definition of terms, symbols and units.
Term Symbol = Definition Unit Comment Cell suspension s s volume of cell suspension s Vs mL The symbol s is used for the sample in a mixture (ce suspended in medium), distinguished from the symbol S for the pure sample. Cell suspension s is the total sample obtained upon harvesting the cells. Frequently the sediment of cells with Vpellet obtained from centrifugation is re-suspended in the experimental medium (e.g. MiR05 plus pyruvate) to obtain Vs = Vpellet + ΔVs. cell count in Vs Nce,s = Cce,s∙Vs Mx The total cell harvest available for respirometry and additional analyses of the sample of cells. cell-count concentration in cell suspension Vs Cce,s = Nce,s/Vs Mx/mL subsample from Vs for respirometry J Vs→J = Vs-Vs→N-Vs→j mL For high accuracy and reproducibility of technical repeats of respiration, Vs→J should be equal or larger than 0.05 mL. Vs→j is the volume of subsamples obtained for assays other than J and N, or a residual subsample. subsample from Vs for cell counting Vs→N mL There is insufficient quantitative evidence for the following consideration: For a reliable cell count and normalization of respiration, Vs→N should be equal or larger than 0.05 mL. Smaller volumes are taken as a subsample for counting, if the aim is merely to define a procedure of dilutions, with final subsamples taken after dilutions for cell-counting. cell count in cell-count subsample from s Nce,s→N = Cce,s∙Vs→N Mx These cells are not available for respirometry, but only a fraction is required for addition into cell counters, hence several technical repeats of cell counting are feasible, or remaining cells may be used for additional characterization of the cell sample. Respirometric stock J J Volume added to Vs→J ΔVJ = VJ-Vs→J mL Volume added for dilution of Cce,s to Cce,J. volume of respirometric stock J VJ = Nce,J/Cce,J mL The respirometric stock is the cell suspension diluted from the total sample Vs to the subsample VJ for further subsampling of cells to be added into the respirometric chamber for measurement of flux J. VJ = Vs→J + ΔVJ cell count in VJ Nce,J = Cce,s∙Vs→J = Nce,s-Nce,s→N-Nce,s→j Mx The cell count available for respirometry. cell-count concentration respirometric stock VJ Cce,J = Mx/mL If cells are pipetted into the O2k-chamber opened after closing and siphoning off any medium from the receptacle of the stopper, then Cce,J is adjusted according to Cce·V'/VJ.i. subsample volume of stock VJ titrated into respirometer chamber VJ.i mL i = 1 to n, where n is the number of technical respirometric repeats. For a reliable cell count and normalization of respiration, VJ.i should be equal or larger than 0.05 mL. maximum number of technical JO2 repeats n = VJ/VJ.i Volume balance and cell suspension in respirometric chamber experimental chamber volume V mL Effectively mixed aqueous volume in the experimental system, which is the closed chamber with the stopper in a volume-calibrated position (2.0 mL or 0.5 mL). When the closed chamber contains respiration medium without sample, a measurement is obtained of instrumental O2 background flux JV,O2° [state (1) in Figure 3b]. Volume-specific respiratory flux JV,O2 of the sample in the closed chamber is the total oxygen flux corrected for instrumental background [state (6) in Figure 3b]. experimental chamber volume plus dead volume in stopper capillary V´ = V + Vstc mL When the stopper is removed from a closed chamber with a completely filled stopper capillary but without any aqueous medium in the receptacle of the stopper, the chamber is said to be 'open'. The volume Vstc in the stopper capillary is drawn into the chamber [state (2) in Figure 3b]. reduced volume in open chamber V´´ = V´ + Vout mL The volume of respiration medium is reduced by Vout (which has a negative value when medium is removed from the chamber) in the open chamber before adding the cell suspension, avoiding a large excess volume leading to loss of cells upon closing the chamber [state (3) in Figure 3b]. total volume in open chamber after addition of cells V# = V + Vstc + Vout + VJ,i mL The minimum V# is 0.54 mL or 2.085 mL in the 0.5-mL or 2.0-mL chamber, respectively [state (4) in Figure 3b]. The volume of the stopper capillary does not mix with V in the closed chamber. Therefore, only V is the experimental volume and VStopper capillary is considered as a 'dead volume' outside of the experimental chamber volume. volume in open chamber after subsampling cell suspension from chamber V´´´ = V# - ΣVi→j mL The minimum V´´´ is 0.54 mL or 2.085 mL in the 0.5-mL or 2.0-mL chamber, respectively [state (5) in Figure 3b]. volume of stopper capillary Vstc mL Vstc = 0.04 mL and 0.07 mL in the 0.5-mL and 2.0-mL O2k-chamber, respectively. volume of respiration medium removed from open chamber Vout = VJ.i - ΣVi→j - Vexc mL The stirrer may be stopped when removing medium from the chamber. Removal of medium minimizes excell volume of cell suspension Vexc. sum of volumes sampled from chamber i before closing ΣVi→j mL For various assays j a volume of cell suspension is sampled from the open chamber i before closing the chamber. excess volume of cell suspension Vexc mL Vexc is wasted in the stopper receptacle upon closing the chamber. cell-count in experimental chamber Nce = N·Uce Mx 106 x = 1 Mx cell-count concentration in experimental chamber Cce = Nce/V Mx/mL 106 x/mL = 1 Mx/mL = 109 x/L = 1 Gx/L cell-count in V# Nce# = Cce·V# Mx Respirometry JO2 O2 flow of the cells in the chamber IO2 pmol·s-1 extensive quantity volume-specific O2 flux, per V JV,O2 = IO2/V pmol·s-1·mL-1 size-specific quantity of experimental system O2 flow per cell IO2/ce = JV,O2/Cce amol·s-1·x-1 extensive quantity volume-specific O2 flux, per Vce JO2/Vce = IO2/ce/Vce amol·s-1·pL-1 size-specific quantity of the cell
Keywords
- »Bioblast links: Chamber volume - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- » Volume
- Units: Liter [L]; 1 L = 1 dm3 = 10-3 m3; 1 mL = 1 cm3; 1 µL = 1 mm3
- » Volume
- On chamber volume
- Components of the experimental O2k-chambers
- » Stirrer-Bar\white PVDF\15x6 mm
- » Stirrer-Bar sV\white PVDF\11.5x6.2 mm
- » Stopper\black PEEK\conical Shaft\central Port
- » Stopper sV\black PEEK\conical Shaft\central Port
- » O-ring\Viton\12.5x1 mm
- » O-ring sV\Viton\9.5x1 mm
- » Volume-Calibration Ring
- » Volume-Calibration Ring sV
- » Stopper-Spacer
- » Cover-Slip\black
- » O2k-Chamber Holder
- » O2k-Chamber Holder sV
- » OroboPOS-Holder
- » OroboPOS-Holder sV
- Maintencance
References: volume
| Bioblast link | Reference | Year |
|---|---|---|
| Bureau International des Poids et Mesures 2019 The International System of Units (SI) | Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216. ISBN 978-92-822-2272-0 | 2019 |
| Gnaiger 2020 BEC MitoPathways | Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | 2020 |
| Gnaiger 2020 MitoFit x | Gnaiger E (2021) The elementary unit — canonical reviewer's comments on: Bureau International des Poids et Mesures (2019) The International System of Units (SI) 9th ed. https://doi.org/10.26124/mitofit:200004.v2 | 2021 |
| BEC 2020.1 doi10.26124bec2020-0001.v1 | Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1 | 2020 |
MitoPedia methods:
Respirometry
MitoPedia O2k and high-resolution respirometry:
DatLab,
Oroboros QM,
O2k-Respirometry,
O2k-FluoRespirometry