Garcia-Roves 2018 MiP2018b
| Generating mitochondrial respirometry reference values from permeabilized mouse soleus muscle fibers. Working Group 2 report. Garcia-Roves_Presentation |
Link: MiP2018
Garcia-Roves PM, Gama-Perez P, Dahdah N, Doerrier C, Gnaiger E, Lemieux H, Holody CD, Carpenter RG, Tepp K, Puurand M, Kaambre T, Dubouchaud H, Chabi B, Cortade F, Ost M, Pesta D, Calabria E, Casado M, Fernandez-Ortiz M, Acuna-Castroviejo D, Villena J, Grefte S, Keijer J, O'Brien KA, Sowton A, Murray AJ, Campbell MD, Marcinek DJ (2018)
Event: MiP2018
The objective of WG2 is the generation of reference values for mitochondrial respirometry in permeabilized skeletal muscle sample preparations. The idea is that new researchers in the field follow a reference protocol and check if their values are in an acceptable range. This approach could serve to test researchers’ technical skills and therefore determine if they are proficient enough to perform their own experiments with confidence.
We initially ran a pilot study including 7 European groups. The results obtained show, as we expected, low variability among laboratories. After some adjustments and identifying some relevant steps that need to be carefully taken into consideration, the approach and results were judged satisfactory. Therefore, last spring the invitation was open to all laboratories worldwide. The final aim is to collect all the data and write a manuscript by 2019.
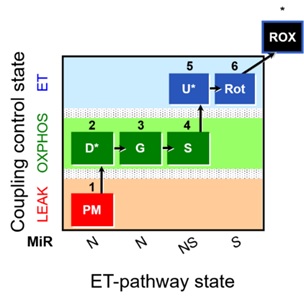
All research groups involved obtained respirometry reference values in permeabilized soleus fibers from males (N=4) and females (N=4) C57BL/6J mice aged 14-16 weeks. The substrate-uncoupler-inhibitor titration (SUIT) protocol used in the study is represented in Figure 1 [1]. The presentation will give detailed information on the experimental procedures followed to fulfill our objective. Finally, we will present and discuss our preliminary data, including recent results from other 8 international laboratories that have already performed the experiments. This presentation should set the stage to initiate a discussion and define future actions of the Working Group 2.
• Bioblast editor: Plangger M
• O2k-Network Lab: ES Barcelona Garcia-Roves PM, AT Innsbruck Oroboros, CA Edmonton Lemieux H, EE Tallinn Kaambre T, FR Montpellier Wrutniak-Cabello C, DE Nuthetal Klaus S, DE Duesseldorf Roden M, IT Verona Calabria E, ES Valencia Casado Pinna M, ES Granada Acuna-Castroviejo D, NL Nijmegen Koopman WJ, NL Wageningen Keijer J, UK Cambridge Murray AJ, US WA Seattle Marcinek DJ, DE Leipzip Ost M
Affiliations
Garcia-Roves PM(1), Gama-Perez P(1), Dahdah N(1), Doerrier C(2), Gnaiger E(2,3), Lemieux H(4), Holody CD(4), Carpenter RG(4), Tepp K(5), Puurand M(5), Kaambre T(5), Dubouchaud H(6), Chabi B(7), Cortade F(7), Ost M(8), Pesta D(9,10), Calabria E(11), Casado M(12), Fernandez-Ortiz M(13), Acuna-Castroviejo D(13), Villena JA(14), Grefte S(15), Keijer J(15), O'Brien KA(16), Sowton A(16), Murray AJ(16), Campbell MD(17), Marcinek DJ(17)
- Dept Physiological Sciences, Univ Barcelona and Bellvitge Biomedical Research Inst, Spain
- Oroboros Instruments
- Dept Visceral, Transplant Thoracic Surgery, Daniel Swarovski Research Lab, Medical Univ Innsbruck; Austria
- Fac Saint-Jean, Univ Alberta, Canada
- Lab Chemical Biology, National Inst Chemical Physics Biophysics, Estonia
- DMEM, Univ Montpellier, INRA, France
- Lab Bioénergétique Fondamentale Appliquée, Univ Grenoble Alpes, INSERM, France
- German Inst Human Nutrition Potsdam-Rehbruecke
- Inst Clinical Diabetology, German Diabetes Center, Leibniz Center Diabetes Research Heinrich-Heine Univ Düsseldorf
- German Center Diabetes Research, Munich, Neuherberg; Germany
- Dept Neurological and Movement Sciences, Univ Verona, Italy
- Dept Molecular and Cellular Pathology and Therapy, Inst Biomedicina Valencia
- Biomedical Research Center, Univ of Granada
- Metabolism and Obesity Lab, Vall d’Hebron Research Inst; Spain
- Human and Animal Physiology, Wageningen Univ, The Netherlands
- Dept Physiology, Development & Neuroscience, Univ Cambridge, UK
- Dept Radiology, Univ Washington, South Lake Union, WA, USA. - [email protected]
Support
- European Union Framework Programme Horizon 2020 COST Action CA15203 MitoEAGLE. Instituto de Salud Carlos III- reference PI15/00701. MitoFit. National Institute of Health-P01 AG001751.
Figures
Figure 1. SUIT protocol. Saturated concentrations of pyruvate (P); malate (M); ADP with MgCl2 (D*), saturated ADP must be tested in each experiment; cytochrome c (c); glutamate (G); succinate (S); ADP (D*): add 2.5 mM to check if ADP is saturated after the addition of succinate; FCCP/CCCP (U*): 0.5 µM steps to reach the maximum non-coupled respiration (ET), report the uncoupler used (FCCP or CCCP); rotenone (Rot); Antimycin A (Ama).
References
Labels: MiParea: Respiration, Instruments;methods
Organism: Mouse
Tissue;cell: Skeletal muscle
Preparation: Permeabilized tissue
Coupling state: OXPHOS, ET
Pathway: N, S, NS, ROX
HRR: Oxygraph-2k
MitoEAGLE