| Komlódi T, Cardoso LHD, Doerrier C, Moore AL, Rich PR, Gnaiger E (2021) Coupling and pathway control of coenzyme Q redox state and respiration in isolated mitochondria. Bioenerg Commun 2021.3. https://doi.org/10.26124/bec:2021-0003 |
» Bioenerg Commun 2021.3. ![]() published online 2021-11-11
published online 2021-11-11
Komlodi Timea, Cardoso Luiza HD, Doerrier Carolina, Moore Anthony L, Rich Peter R, Gnaiger Erich (2021) Bioenerg Commun
Abstract: ![]() doi:10.26124/bec:2021-0003
doi:10.26124/bec:2021-0003
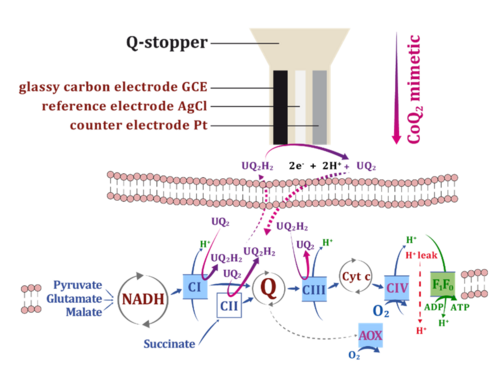
Redox states of the mitochondrial coenzyme Q pool, which reacts with the electron transfer system, reflect the balance between (1) reducing capacities of electron flow from fuel substrates converging at the Q-junction, (2) oxidative capacities downstream of Q to O2, and (3) the load on the OXPHOS system utilizing or dissipating the protonmotive force.
A three-electrode sensor (Rich 1988; Moore et al 1988) was implemented into the NextGen-O2k to monitor continuously the redox state of CoQ2 added as a Q-mimetic simultaneously with O2 consumption. The Q-Module was optimized for high signal-to-noise ratio, minimum drift, and minimum oxygen diffusion. CoQ2 equilibrates in the same manner as Q at Complexes CI, CII and CIII. The CoQ2 redox state is monitored amperometrically with the working electrode, which is poised at CoQ2 redox peak potentials determined by cyclic voltammetry. The voltammogram also provides quality control of the Q-sensor and reveals chemical interferences.
The CoQ2 redox state and O2 consumption were measured simultaneously in isolated mouse cardiac and brain mitochondria. CoQ2 ― and by implication mitochondrial Q ― was more oxidized when O2 flux was stimulated by coupling control: when energy demand increased from LEAK to OXPHOS and electron transfer capacities in the succinate pathway. In contrast, CoQ2 was more reduced when O2 flux was stimulated by pathway-control of electron input capacities, increasing from the NADH (N)- to succinate (S)-linked pathway which converge at the Q-junction, with CI-Q-CIII and CII-Q-CIII segments, respectively. N- and S- respiratory pathway capacities were not completely additive, compatible with partitioning of Q intermediary between the solid-state and liquid-state models of supercomplex organization. The direct proportionality of CoQ2 reduction and electron input capacities through the CI-Q-CIII and CII-Q-CIII segments suggests that CoQ2 is accurately mimicking mitochondrial Q-redox changes.
• Keywords: coenzyme Q CoQ, Q-junction, Q-redox state, electron transfer system ETS, ETS-reactive Q-pool Q, mitochondrial coenzyme Q mtCoQ, supercomplexed Q, free Q-pool according to the fluid-state model Qfree, cyclic voltammetry CV, high-resolution respirometry HRR, isolated mitochondria imt, mouse heart mitochondria, mouse brain mitochondria, oxygen consumption, SUIT protocols, coupling control, pathway control, NS-pathway, additivity
• Bioblast editor: Gnaiger E, Cardoso LHD
• O2k-Network Lab: AT Innsbruck Oroboros
ORCID: ![]() Komlodi Timea,
Komlodi Timea,
![]() Cardoso Luiza HD,
Cardoso Luiza HD,
![]() Doerrier Carolina,
Moore Anthony L,
Doerrier Carolina,
Moore Anthony L,
![]() Rich Peter R,
Rich Peter R,
![]() Gnaiger Erich
Gnaiger Erich
Video - NextGen-O2k: the Q-Module
Data availability
- Original files are available Open Access at Zenodo repository: 10.5281/zenodo.4478400
Support
- Supported by project NextGen-O2k which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 859770.
Keywords
- Bioblast links: Q - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Coenzyme Q
- » Coenzyme Q
- » Quinone, Ubiquinone Q; oxidized
- » Quinol, Ubiquinol QH2; reduced
- » Semiquinone
- » Coenzyme Q2
- » Q-redox state
- » Q-pools
- Coenzyme Q
- Mitochondrial pathways, respiratory Complexes, and Q
- » Q-cycle
- » Q-junction
- » Convergent electron flow
- » NS-pathway
- » FNS
- » FNSGp
- Mitochondrial pathways, respiratory Complexes, and Q
- NextGen-O2k and Q-Module
References
| Link | Reference | Year | View |
|---|---|---|---|
| Aberg 1992 Arch Biochem Biophys | Aberg F, Appelkvist EL, Dallner G, Ernster L (1992) Distribution and redox state of ubiquinones in rat and human tissues. Arch Biochem Biophys 295:230-4. | 1992 | PMID:1586151 |
| Alcázar-Fabra 2016 Biochim Biophys Acta | Alcázar-Fabra M, Navas P, Brea-Calvo G (2016) Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim Biophys Acta 1857:1073-1078. | 2016 | PMID:26970214 Open Access |
| Ausili 2008 J Phys Chem B | Ausili A, Torrecillas A, Aranda F, de Godos A, Sánchez-Bautista S, Corbalán-García S, Gómez-Fernández JC (2008) Redox state of coenzyme Q10 determines its membrane localization. J Phys Chem B 112:12696-702. | 2008 | PMID:18795772 |
| Awad 2018 Essays Biochem | Awad AM, Bradley MC, Fernandez-Del-Rio L, Nag A, Tsui HS, Clarke CF (2018) Coenzyme Q10 deficiencies: pathways in yeast and humans. Essays Biochem 62:361-76. | 2018 | PMID:29980630 Open Access |
| Balaban 2005 Cell | Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120:483-95. https://doi.org/10.1016/j.cell.2005.02.001 | 2005 | PMID:15734681 Open Access |
| Bentinger 2007 Mitochondrion | Bentinger M, Brismar K, Dallner G (2007) The antioxidant role of coenzyme Q. Mitochondrion Suppl:S41-50. | 2007 | PMID:17482888 |
| Bentinger 2010 Biochem Biophys Res Commun | Bentinger M, Tekle M, Dallner G (2010) Coenzyme Q--biosynthesis and functions. Biochem Biophys Res Commun 396:74-9. | 2010 | PMID:20494114 |
| Bianchi 2003 Biofactors | Bianchi C, Fato R, Genova ML, Parenti Castelli F, Lenaz G (2003) Structural and functional organization of Complex I in the mitochondrial respiratory chain. Biofactors 18:3-9. | 2003 | PMID:14695915 |
| Bianchi 2004 J Biol Chem | Bianchi C, Genova ML, Parenti Castelli G, Lenaz G (2004) The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem 279:36562-9. | 2004 | PMID: 15205457 Open Access |
| Cohen 2008 IUPAC Green Book | Cohen ER, Cvitas T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor HL (2008) Quantities, Units and Symbols in Physical Chemistry. IUPAC Green Book 3rd Edition, 2nd Printing, IUPAC & RSC Publishing, Cambridge. | 2008 | Open Access |
| Cottingham 1983 Biochim Biophys Acta | Cottingham IR, Moore AL (1983) Ubiquinone pool behaviour in plant mitochondria. Biochim Biophys Acta 724:191-200. | 1983 | [1] |
| Crane 1957 Biochim Biophys Acta | Crane FL, Hatefi Y, Lester RL, Widmer C (1957) Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta 1000:362-3. | 1957 | PMID:2673386 |
| Crane 1985 Biochim Biophys Acta | Crane FL, Sun IL, Clark MG, Grebing C, Low H (1985) Transplasma-membrane redox systems in growth and development. Biochim Biophys Acta 811:233-64. | 1985 | PMID:3893544 |
| Crane 1959 Biochim Biophys Acta | Crane FL, Widmer C, Lester RL, Hatefi Y, Fechner W (1959) Studies on the electron transport system: XV. Coenzyme Q (Q275) and the succinoxidase activity of the electron transport particle. Biochim Biophys Acta 31:476-89. | 1959 | [2] |
| Crofts 2004 Annu Rev Physiol | Crofts AR (2004) The cytochrome bc1 complex: function in the context of structure. Annu Rev Physiol 66:689-733. | 2004 | PMID:14977419 |
| Doerrier 2018 Methods Mol Biol | Doerrier C, Garcia-Souza LF, Krumschnabel G, Wohlfarter Y, Mészáros AT, Gnaiger E (2018) High-Resolution FluoRespirometry and OXPHOS protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol Biol 1782:31-70. https://doi.org/10.1007/978-1-4939-7831-1_3 | 2018 | PMID: 29850993 » |
| Dry 1989 Arch Biochem Biophys | Dry IB, Moore AL, Day DA, Wiskich JT (1989) Regulation of alternative pathway activity in plant mitochondria: nonlinear relationship between electron flux and the redox poise of the quinone pool. Arch Biochem Biophys 273:148-57. | 1989 | PMID: 2757390 |
| Echtay 2000 Nature | Echtay KS, Winkler E, Klingenberg M (2000) Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature 408:609-13. | 2000 | PMID:11117751 |
| Enriquez 2014 Mol Syndromol | Enriquez JA, Lenaz G (2014) Coenzyme Q and the respiratory chain: coenzyme Q pool and mitochondrial supercomplexes. Mol Syndromol 5:119-40. | 2014 | PMID: 25126045 Open Access |
| Ernster 1969 Eur J Biochem | Ernster L, Lee IY, Norling B, Persson B (1969) Studies with ubiquinone-depleted submitochondrial particles. Essentiality of ubiquinone for the interaction of succinate dehydrogenase, NADH dehydrogenase, and cytochrome b. Eur J Biochem 9:299-310. | 1969 | PMID: 4307591 Open Access |
| Estornell 1992 FEBS Letters | Estornell E, Fato R, Castelluccio C, Cavazzoni M, Parenti Castelli G, Lenaz G (1992) Saturation kinetics of coenzyme Q in NADH and succinate oxidation in beef heart mitochondria. FEBS Letters 311:107-9. | 1992 | |
| Fazakerley 2018 Elife | Fazakerley 1DJ, Chaudhuri R, Yang P, Maghzal GJ, Thomas KC, Krycer JR, Humphrey SJ,Parker BL, Fisher-Wellman KH, Meoli CC, Hoffman NJ, Diskin C, Burchfield JG, Cowley MJ, Kaplan W, Modrusan Z, Kolumam G, Yang JY, Chen DL, Samocha-Bonet D, Greenfield JR, Hoehn KL, Stocker R, James DE (2018) Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance. Elife 7:32111. | 2018 | PMID:29402381 Open Access |
| Fontaine 1998 J Biol Chem | Fontaine E, Ichas F, Bernardi P (1998) A ubiquinone-binding site regulates the mitochondrial permeability transition pore. J Biol Chem 273:25734-40. | 1998 | PMID:9748242 |
| Gille 2000 Arch Biochem Biophys | Gille L, Nohl H (2000) The existence of a lysosomal redox chain and the role of ubiquinone. Arch Biochem Biophys 375:347-54. | 2000 | PMID:10700391 |
| Gnaiger 2001 Respir Physiol | Gnaiger E (2001) Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. https://doi.org/10.1016/S0034-5687(01)00307-3 | 2001 | Respir Physiol 128:277-97. PMID: 11718759 |
| Gnaiger 2008 POS | Gnaiger E (2008) Polarographic oxygen sensors, the oxygraph and high-resolution respirometry to assess mitochondrial function. In: Mitochondrial dysfunction in drug-induced toxicity (Dykens JA, Will Y, eds) John Wiley & Sons, Inc, Hoboken, NJ:327-52. | 2008 | |
| Gnaiger 2020 BEC MitoPathways | Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | 2020 | |
| Gnaiger 2021 MitoFit BCA | Gnaiger E (2021) Bioenergetic cluster analysis – mitochondrial respiratory control in human fibroblasts. MitoFit Preprints 2021.08. https://doi.org/10.26124/mitofit:2021-0008 | 2021 | MitoFit Preprints 2021.08. Bioenergetic cluster analysis – mitochondrial respiratory control in human fibroblasts
|
| Gnaiger 2000 Life in the Cold | Gnaiger E, Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Steurer W, Margreiter R (2000) Mitochondria in the cold. In: Life in the Cold (Heldmaier G, Klingenspor M, eds) Springer, Berlin, Heidelberg:431-42. https://doi.org/10.1007/978-3-662-04162-8_45 | 2000 | |
| Goldstein 1990 Nature | Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425-30. | 1990 | PMID:1967820 |
| González-Mariscal 2014 IUBMB Life | González-Mariscal I, García-Testón E, Padilla S, Martín-Montalvo A, Pomares-Viciana T, Vazquez-Fonseca L, Gandolfo-Domínguez P, Santos-Ocaña C (2014) Regulation of coenzyme Q biosynthesis in yeast: a new complex in the block. IUBMB Life 66:63-70. | 2014 | PMID:24470391 Open Access |
| Graham 2018 Standard operating procedures for cyclic voltammetry | Graham D (2018) Standard operating procedures for cyclic voltammetry. ISBN 978-1-387-51430-4. | 2018 | http://sop4cv.com/ |
| Gulaboski 2016 J Solid State Electrochem | Gulaboski R, Markovski V, Jihe Z (2016) Redox chemistry of coenzyme Q—a short overview of the voltammetric features. J Solid State Electrochem 20:3229–3238. | 2016 | [3] |
| Gutman 1985 Coenzyme Q, Chichester, UK: Wiley | Gutman M (1985) Kinetic analysis of electron flux through the mitochondrial system. Coenzyme Q, Chichester, UK: Wiley 10:215-34. | 1985 | [4] |
| Gvozdjakova 2021 PLoS One | Gvozdjáková A, Sumbalová Z, Kucharská J, Szamosová M, Čápová L, Rausová Z, Vančová O, Mojto V, Langsjoen P, Palacka P (2021) Platelet mitochondrial respiration and coenzyme Q10 could be used as new diagnostic strategy for mitochondrial dysfunction in rheumatoid diseases. PLoS ONE 16:e0256135. | 2021 | PMID: 34582480 Open Access |
| Gvozdjakova 2020 Diagnostics (Basel) | Gvozdjáková A, Sumbalová Z, Kucharská J, Komlósi M, Rausová Z, Vančová O, Számošová M, Mojto V (2020) Platelet mitochondrial respiration, endogenous coenzyme Q10 and oxidative stress in patients with chronic kidney disease. Diagnostics (Basel) 10:176. | 2020 | PMID: 32210203 Open Access » |
| Hackenbrock 1986 J Bioenerg Biomembr | Hackenbrock CR, Chazotte B, Gupte SS (1986) The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr 18:331–68. | 1986 | PMID:3021714 |
| Hatefi 1959 Biochim Biophys Acta | Hatefi Y, Lester RL, Crane FL, Widmer C (1959) Studies on the electron transport system. XVI. Enzymic oxidoreduction reactions of coenzyme Q. Biochim Biophys Acta 31:490-501. | 1959 | PMID:13628678 |
| Hernandez-Camacho 2018 Front Physiol | Hernández-Camacho JD, Bernier M, López-Lluch G, Navas P (2018) Coenzyme Q10 Supplementation in aging and disease. Front Physiol 9:44. | 2018 | PMID:29459830 |
| Hunte 2003 FEBS Letters | Hunte C, Palsdottir H, Trumpower BL (2003) Protonmotive pathways and mechanisms in the cytochrome bc1 complex. FEBS Letters 545:39-46. | 2003 | PMID:12788490 |
| Joergensen 1985 Biochem J | Jørgensen BM, Rasmussen HN, Rasmussen UF (1985) Ubiquinone reduction pattern in pigeon heart mitochondria. Identification of three distinct ubiquinone pools. Biochem J 229:621-9. | 1985 | PMID:4052014 Open Access |
| Kalen 1987 Acta Chem Scand B | Kalén A, Appelkvist EL, Dallner G (1987) Biosynthesis of ubiquinone in rat liver. Acta Chem Scand B 41:70-2. | 1987 | PMID:3577554 |
| Kroeger 1966 Biochem Z | Kröger A, Klingenberg M (1966) On the role of ubiquinone in mitochondria. II. Redox reactions of ubiquinone under the control of oxidative phosphorylation. Biochem Z 344:317-36. | 1966 | PMID:4295856 |
| Kroeger 1973b Eur J Biochem | Kröger A, Klingenberg M (1973) Further evidence for the pool function of ubiquinone as derived from the inhibition of the electron transport by antimycin. Eur J Biochem 39:313-23. | 1973 | PMID:4359626 Open Access |
| Kroeger 1973 Eur J Biochem | Kröger A, Klingenberg M (1973) The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur J Biochem 34:358-68. | 1973 | PMID:4351161 Open Access |
| MiPNet20.07 IsolationRatBrain-mt | Laboratory Protocol: isolation of rat brain mitochondria. | 2016-06-18 | |
| Lemieux 2017 Sci Rep | Lemieux H, Blier PU, Gnaiger E (2017) Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep 7:2840. doi:10.1038/s41598-017-02789-8 | 2017 | PMID: 28588260 Sci Rep Open Access |
| Lenaz 2009 Int J Biochem Cell Biol | Lenaz G, Genova ML (2009) Structural and functional organization of the mitochondrial respiratory chain: A dynamic super-assembly. Int J Biochem Cell Biol 41:1750-72. | 2009 | PMID:19711505 Open Access |
| Lopez-Lluch 2010 Mech Ageing Dev | Lopez-Lluch G, Rodriguez-Aguilera JC, Santos-Ocana C, Navas P (2010) Is coenzyme Q a key factor in aging? Mech Ageing Dev 131:225-35. | 2010 | PMID:20193705 |
| Lowry 1951 J Biol Chem | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265-75. | 1951 | PMID:14907713 Open Access |
| Miles 2007 Mitochondrion | Miles MV (2007) The uptake and distribution of coenzyme Q(10). Mitochondrion 7 Suppl:S72-7. | 2007 | PMID:17446143 |
| Mitchell 1961 Nature | Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144-8. | 1961 | PMID: 13771349 |
| Mitchell 1975 FEBS Letters | Mitchell P (1975) The protonmotive Q cycle: A general formulation. FEBS Lett 59:137-9. | 1975 | Open Access |
| Moore 1991 Plant Physiol | Moore AL, Dry IB, Wiskich JT (1991) Regulation of electron transport in plant mitochondria under state 4 conditions. Plant Physiol 95:34-40. | 1991 | PMID: 16667977 Open Access |
| Moore 1988 FEBS Letters | Moore AL, Dry IB, Wiskich TJ (1988) Measurement of the redox state of the ubiquinone pool in plant mitochondria. FEBS Lett 235:76-80. | 1988 | Open Access |
| Morre 1989 Biotechniques | Morré DJ, Morré DM (1989) Preparation of mammalian plasma membranes by aqueous two-phase partition. Biotechniques 7:946-58. | 1989 | PMID:2483665 |
| Morre 2011 Biofactors | Morré DJ, Morré DM (2011) Non-mitochondrial coenzyme Q. Biofactors 37:355-60. | 2011 | PMID:21674641 |
| Noh 2013 Cell Death Dis | Noh YH, Kim K-Y, Shim MS, Choi S-H, Choi S, Ellisman MH, Weinreb RN, Perkins GA, Ju W-K (2013) Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis 4:e820. | 2013 | PMID:24091663 Open Access |
| Nyquist 1970 Biochim Biophys Acta | Nyquist SE, Barr R, Morré DJ (1970) Ubiquinone from rat liver Golgi apparatus fractions. Biochim Biophys Acta 208:532-4. | 1970 | PMID:5506581 |
| Osakai 2019 Electrochemistry | Osakai T, Yamamoto T, Ueki M (2019) Directional electron transfer from ubiquinone-10 to cytochrome c at a biomimetic self-assembled monolayer modified electrode. Electrochemistry 87:59-64. | 2019 | Open Access |
| Petrova 2014 Proc Chem | Petrova EV, Korotkova EI, Kratochvil B, Voronova OA, Dorozhko EV, Bulycheva EV (2014) Investigation of coenzyme Q10 by voltammetry. Proc Chem 10:173-8. https://doi.org/10.1016/j.proche.2014.10.030. | 2014 | Open Access |
| Rauchova 1997 Arch Biochem Biophys | Rauchová H, Fato R, Drahota Z, Lenaz G (1997) Steady-state kinetics of reduction of coenzyme Q analogs by glycerol-3-phosphate dehydrogenase in brown adipose tissue mitochondria. Arch Biochem Biophys 344:235-41. | 1997 | PMID:9244403 Open Access |
| Reed 1987 Biochem J | Reed JS, Ragan CI (1987) The effect of rate limitation by cytochrome c on the redox state of the ubiquinone pool in reconstituted NADH: cytochrome c reductase. Biochem J 247:657-62. | 1987 | PMC:1148462 |
| Rich 1982 Faraday Discuss Chem Soc | Rich PR (1982) Electron and proton transfers in chemical and biological quinone systems. Faraday Discuss Chem Soc 74:349-64. | 1982 | PMID:7183455 |
| Rich 1984 Biochim Biophys Acta | Rich PR (1984) Electron and proton transfers through quinones and cytochrome bc complexes. Biochim Biophys Acta 768:53-79. | 1984 | PMID: 6322844 |
| Rich 1988 Glynn Res. Ph. | Rich PR (1988) Patent of Q-electrode. Glynn Res. Ph. European Patent no.85900699.1. | 1988 | |
| Rich 2004 Biochim Biophys Acta | Rich PR (2004) The quinone chemistry of bc complexes. Biochim Biophys Acta 1658:165-71. | 2004 | PMID:15282188 Open Access |
| Rodriguez-Hernandez 2009 Autophagy | Rodríguez-Hernández A, Cordero MD, Salviati L, Artuch R, Pineda M, Briones P, Izquierdo LG, Cotán D, Navas P, Sánchez-Alcázar JA (2009) Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy 5:19-32. | 2009 | PMID: 19115482 Open Access |
| Sastry 1961 Nature | Sastry PS, Jayaraman J, Ramasarma T (1961) Distribution of coenzyme Q in rat liver cell fractions. Nature 189:577. | 1961 | Open Access |
| Song 2011 Free Radical Biol Med | Song Y, Buettner GR (2011) Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radical Biol Med 919-62. | 2011 | PMID: 20493944 Open Access |
| Stefely 2018 Trends Biochem Sci | Stefely JA, Pagliarini DJ (2018) Biochemistry of Mitochondrial Coenzyme Q Biosynthesis. Trends Biochem Sci 42: 824–843. | 2018 | PMID:28927698 Open Access |
| Stoner 1984 J Bioenerg Biomembr | Stoner CD (1984) Steady-state kinetics of the overall oxidative phosphorylation reaction in heart mitochondria. Determination of the coupling relationships between the respiratory reactions and miscellaneous observations concerning rate-limiting steps. J Bioenerg Biomembr 16:115-41. | 1984 | PMID:6100296 |
| Takada 1984 Methods Enzymol | Takada M, Ikenoya S, Yuzuriha T, Katayama K (1984) Simultaneous determination of reduced and oxidized ubiquinones. Methods Enzymol 105:147-55. | 1984 | |
| Tang 2012 Methods Mol Biol | Tang PH, Miles MV (2012) Measurement of oxidized and reduced coenzyme Q in biological fluids, cells, and tissues: an HPLC-EC method. Methods Mol Biol 837:149-68. | 2012 | PMID:22215546 |
| Tran 2007 Mitochondrion | Tran UC, Clarke CF (2007) Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion 7 Suppl:S62-71. | 2007 | PMID:17482885 Open Access |
| Trumpower 1990 J Biol Chem | Trumpower BL (1990) The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem 265:11409-12. | 1990 | PMID:2164001 |
| Trumpower 1994 Annu Rev Biochem | Trumpower BL, Gennis RB (1994) Energy transduction by cytochrome complexes in mitochondrial and bacterial respiration: the enzymology of coupling electron transfer reactions to transmembrane proton translocation. . Annu Rev Biochem 63:675-716. | 1994 | PMID:7979252 |
| Turunen 2004 Biochim Biophys Acta | Turunen M, Olsson J, Dallner G (2004) Metabolism and function of coenzyme Q. Biochim Biophys Acta 1660:171-99. | 2004 | PMID:14757233 Open Access |
| Urban 1969 Eur J Biochem | Urban PF, Klingenberg M (1969) On the redox potentials of ubiquinone and cytochrome b in the respiratory chain. Eur J Biochem 9:519-25. | 1969 | Open Access |
| Van den Bergen 1994 Eur J Biochem | Van den Bergen CW, Wagner AM, Krab K, Moore AL (1994) The relationship between electron flux and the redox poise of the quinone pool in plant mitochondria. Interplay between quinol-oxidizing and quinone-reducing pathways. Eur J Biochem 226:1071-8. | 1994 | PMID: 7813462 Open Access |
| Watts 2017 Genetics | Watts JL, Ristow M (2017) Lipid and Carbohydrate Metabolism in Caenorhabditis elegans. Genetics 207:413-46. | 2017 | PMID:28978773 Open Access |
| Wolf 1958 J Am Chem Soc | Wolf DE, Hoffman CH, Trenner NR, Arison BH, Shunk CH, Linn BO, McPherson JF, Folkers K (1958) Coenzyme Q. I. Structure studies on the coenzyme Q group. J Am Chem Soc 80:4752. | 1958 | Open Access |
| Zannoni 1990 FEBS Lett | Zannoni D, Moore AL (1990) Measurement of the redox state of the ubiquinone pool in Rhodobacter capsulatus membrane fragments. FEBS Lett 271:123-7. | 1990 | PMID: 2171997 Open Access |
| MiPNet22.10 MiR05-Kit | Mitochondrial Respiration Medium - MiR05-Kit. | 2022-03-03 | |
| MiPNet24.12 NextGen-O2k: Q-Module | NextGen-O2k: Q-Module manual | 2021-10-29 | |
| MiPNet20.06 IsolationMouseHeart-mt | Laboratory protocol: isolation of mouse heart mitochondria. | 2021-08-09 |
Preprint
Cited by
- Jarmuszkiewicz W, Dominiak K, Budzinska A, Wojcicki K, Galganski L (2023) Mitochondrial coenzyme Q redox homeostasis and reactive oxygen species production. - »Bioblast link«
- Gnaiger E (2021) Beyond counting papers – a mission and vision for scientific publication. Bioenerg Commun 2021.5. https://doi:10.26124/BEC:2021-0005
- Komlodi et al (2022) Hydrogen peroxide production, mitochondrial membrane potential and the coenzyme Q redox state measured at tissue normoxia and experimental hyperoxia in heart mitochondria. MitoFit Preprints 2021 (in prep)
- Komlódi T, Schmitt S, Zdrazilova L, Donnelly C, Zischka H, Gnaiger E. Oxygen dependence of hydrogen peroxide production in isolated mitochondria and permeabilized cells. MitoFit Preprints (in prep).
- Komlodi et al (2022) Hydrogen peroxide production, mitochondrial membrane potential and the coenzyme Q redox state measured at tissue normoxia and experimental hyperoxia in heart mitochondria. MitoFit Preprints 2021 (in prep)
- Gnaiger E (2021) Beyond counting papers – a mission and vision for scientific publication. Bioenerg Commun 2021.5. https://doi:10.26124/BEC:2021-0005
Labels: MiParea: Respiration, Instruments;methods
Organism: Mouse
Tissue;cell: Heart, Nervous system
Preparation: Isolated mitochondria
Regulation: Redox state, Q-junction effect Coupling state: LEAK, OXPHOS, ET Pathway: N, S, NS HRR: Oxygraph-2k, NextGen-O2k, O2k-Protocol
Additivity, SUIT-006 Q mt D071, SUIT-006 Q ce-pce D073, SUIT-031 Q mt D072, SUIT-031 Q ce-pce D074, BEC2021.5, MitoFit 2022 NADH, MitoFit 2021 AmR, Redox, O2k-Demo