Sumbalova 2016a Abstract MitoFit Science Camp 2016
| Optimizing strategies on the malate concentration in SUIT protocols. |
Link:
Sumbalova Z, Krumschnabel G, Doerrier C, Gnaiger E (2016)
Event: MitoFit Science Camp 2016 Kuehtai AT
Substrate-uncoupler-inhibitor-titration (SUIT) protocols used in high-resolution respirometry have been developed [1] to evaluate the control of separate and combined mitochondrial pathways for OXPHOS analysis in an economical approach while at the same time minimizing experimental variability and sample requirement. Nevertheless, there are some inherent limitations to this approach: a) CI-linked respiratory activity cannot be totally isolated from that of CII, unless CII is selectively inhibited. That is, many substrates or combinations of substrates aimed at fuelling CI via feeding into the tricarboxylic acid (TCA) cycle will, besides stimulating NADH production, also produce succinate (S) feeding CII. The extent of the contribution of CII to the overall respiratory activity will depend on the substrate combination chosen, their concentrations, and the cell or tissue-type investigated, reflecting tissue-specific enzyme expression patterns and substrate preferences. b) The activity of CII is prone to be altered by intermediates obtained in the course of evaluation of CI activity. Succinate dehydrogenase (SDH) is known to be sensitive to inhibition by oxaloacetate [2], which may not be added directly but may be produced upon addition of other substrates [3]. Observation on multiple types of mitochondria revealed that malate (M), a substrate used to fuel CI-linked respiration, inhibits both coupled and uncoupled CII-linked respiration [4].
For diagnostic purposes the use in the SUIT protocols of a combination of substrates supporting the highest rates of CI-linked respiration that is free of CII-linked respiration, as well as a proper evaluation of CII-linked respiration is important.
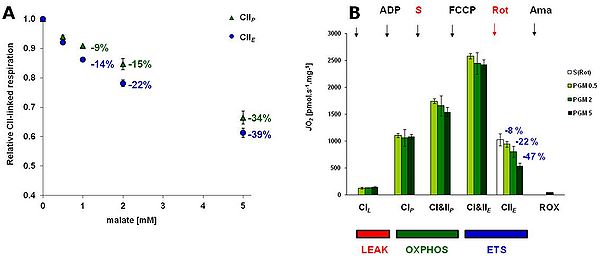
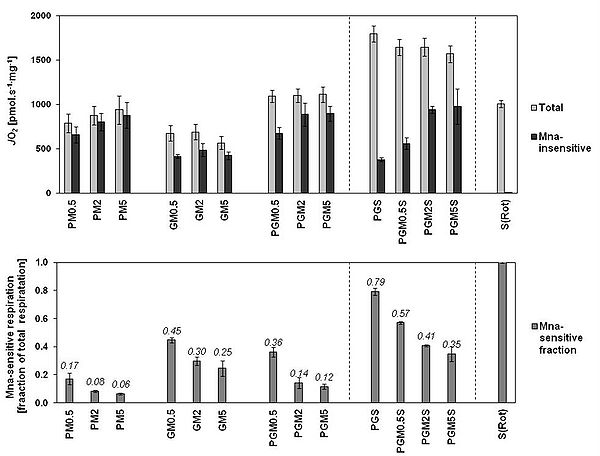
In the present study, we evaluated the effect of three concentrations of M on OXPHOS and ET-pathway respiration of rat brain mitochondria with 10 mM succinate + 0.5 µM rotenone (CII protocol) as well as on the respiratory rates obtained in a comprehensive SUIT protocol using sequential titrations of PMG, ADP, S, FCCP, Rot and Ama (Fig.1). To determine the optimum concentration of M as well as optimal combination of substrates for evaluating CI-linked respiration, we used 0.5, 2 and 5 mM M in combination with P, G, PG and PGS. Towards the end of each run we added 5 mM malonate (Mna), an inhibitor of CII, so as to reveal the actual contribution of CI-linked respiration to total OXPHOS respiration (Fig.2).
• O2k-Network Lab: AT Innsbruck Oroboros, SK Bratislava Sumbalova Z
Affiliations
1-Pharmacobiochem Lab, Fac Med, Comenius Univ, Bratislava, Slovakia; 2-Oroboros Instruments; 3-Dept Visceral, Transplant Thoracic Surgery, D. Swarovski Research Lab, Medical Univ Innsbruck, Austria. – [email protected]
Abstract continued
In rat brain mitochondria, M inhibited CII-linked ET-pathway respiration slightly more than CII-linked OXPHOS respiration (-22 and -15% respectively in 2 mM M) (Fig. 1A). In the comprehensive SUIT protocol, the inhibitory effect of M on SDH became only evident at CII-linked ET-pathway respiration (Fig 1B). The relative share of CI-linked respiration in total respiration with all substrate combinations was highest with 5 mM M (Fig 2A). The combination of P (5 mM) and M (2 or 5 mM) best reflected the maximum capacity of CI-linked respiration with the least involvement of CII-linked respiration (less than 8%). Addition of G (10 mM) to this combination increased total respiration (Fig 2A), but this apparent increase reflected just higher involvement of CII-linked respiration (Fig 2B).
For the proper evaluation of CII-linked ET-pathway respiration, the inhibitory effect of 2 mM M could be overcome by additional titration of succinate (to a final concentration of 50 mM) after rotenone. The inhibition caused by 5 mM M could not be overcome by addition of S.
Based on these data, we suggest using 2 mM M in the SUIT protocols. It appears that 50 mM S could be generally applicable as a single titration in the SUIT protocols right after CI-linked substrates as it does not negatively affect the CI&II-linked OXPHOS and ET-pathway respiratory rates. Nevertheless, we suggest testing the effect of 50 mM S on these rates in each type of preparation before using it routinely in the SUIT protocol.
Figures
'Figure 1. A: The effect of malate on the respiration with 10 mM succinate + 0.5 µM rotenone (CII protocol) in OXPHOS and ET-pathway state in rat brain mitochondria.
B: Respiratory rates determined with the comprehensive protocol with 3 concentrations of malate (M), 0.5, 2 and 5 mM, in mitochondrial respiration medium MiR05 , at 37°C. In the protocol, 5 mM pyruvate (P), 10 mM glutamate (G), M, 2 mM ADP, 10 mM succinate (S) and FCCP 0.5-1 µM were titrated to determine ET capacity with CI&II substrates. Subsequently, 0.5 µM rotenone was added for evaluation of CII-linked ET-pathway, followed by antimycin A for evaluation of residual oxygen consumption (ROX). For comparison, the value of CII-linked ET-pathway determined in the CII protocol is shown in the graph. All data are corrected for ROX shown in the graph and are means ± SEM of experiments conducted with 3-4 preparations.
Figure 2. A: The respiration in OXPHOS state determined in rat brain mitochondria with CI-linked substrates, CI&II-linked substrate combinations, and CII-linked substrate, and the respective malonate-insensitive part obtained after addition of 5 mM malonate. B: The malonate-sensitive part of respiration determined in A expressed as fraction of total OXPHOS respiration. Data are ROX-corrected and are means ± SEM of experiments conducted on 11 preparations with minimum number of experiments for each substrate combination being 3.
References and Support
- Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibres from small biopsies of human muscle. Methods Mol Biol 810:25-58.
- Ackrell BA, Kearney EB (1974) Mayr M. Role of oxalacetate in the regulation of mammalian succinate dehydrogenase. J Biol Chem 249:2021-7.
- Moser MD, Matsuzaki S, Humphries KM (2009) Inhibition of succinate-linked respiration and complex II activity by hydrogen peroxide. Arch Biochem Biophys 48:69-75.
- Sumbalova Z, Vancova O, Krumschnabel G, Gnaiger E (2014) Optimization of malate concentration for high-resolution respirometry: mitochondria from rat liver and brain. Mitochondr Physiol Network 19.13:p37.
Supported by Erasmus and Action Austria-Slovakia
Labels: MiParea: Respiration
Organism: Rat
Tissue;cell: Nervous system
Regulation: Substrate
Coupling state: OXPHOS, ET
Pathway: N, S, NS
HRR: Oxygraph-2k
Event: A1
MitoFit Science Camp 2016