- high-resolution terminology - matching measurements at high-resolution
Malate-anaplerotic pathway control state
Description
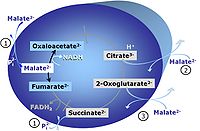
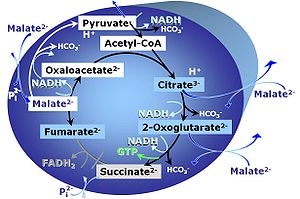
M: Malate alone does not support respiration of mt-preparations if oxaloacetate cannot be metabolized further in the absence of a source of acetyl-CoA. Transport of oxaloacetate across the inner mt-membrane is restricted particularly in liver. Mitochondrial citrate and 2-oxoglutarate (α-ketoglutarate) are depleted by antiport with malate. Succinate is lost from the mitochondria through the dicarboxylate carrier. OXPHOS capacity with malate alone is only 1.3% of that with Pyruvate&Malate in isolated rat skeletal muscle mitochondria. However, many mammalian and non-mammalian mitochondria have a mt-isoform of NADP+- or NAD(P)+-dependent malic enzyme (mtME), the latter being particularly active in proliferating cells. Then the anaplerotic pathway control state with malate alone (aN) supports high respiratory activities comparable to the NADH-linked pathway control states (N) with pyruvate&malate or glutamate&malate substrate combinations (PM-pathway control state, GM-pathway control state).
Abbreviation: M
Reference: Gnaiger 2020 BEC MitoPathways
Communicated by Gnaiger E 2016-01-24, edited by Cardoso LHD and Doerrier C (last update 2020-04-30)
ML
MP
- If malate-anaplerotic pathways are present, malate alone may provide mt-respiration values comparable to OXPHOS state (P). However, to ensure OXPHOS state measurement, a combination of NADH-linked substrates should be performed (i.e. PM, GM, PGM). SUIT-027 evaluates malate-anaplerotic pathway (malate alone in the presence of ADP) and OXPHOS state (P) with pyruvate&malate and pyruvate&glutamate&malate as NADH-linked substrates:
- DL-Protocol for isolated mitochondria and tissue homogenate (mt): SUIT-027 O2 ce-pce D065
ME
MitoPedia concepts: SUIT state