Difference between revisions of "Talk:MitoPedia: Terms and abbreviations"
| Line 1: | Line 1: | ||
== Summary of Core Energy Metabolism lecture series == | |||
[http://www.bioblast.at/index.php?title=Talk:MitoCom_Network&action=purge#Core_Energy_Metabolism_-_an_interactive_lecture_series|Core Energy Metabolism lecture series] | [http://www.bioblast.at/index.php?title=Talk:MitoCom_Network&action=purge#Core_Energy_Metabolism_-_an_interactive_lecture_series|Core Energy Metabolism lecture series] | ||
Revision as of 10:15, 15 March 2013
Summary of Core Energy Metabolism lecture series
Energy Metabolism lecture series
Core Energy Metabolism implies processes such as Respiration and Fermentation, both of which are involved in ATP production.
Respiration is the process of oxidative phosphorylation, where a chemisosmotic potential drives ATP synthesis.
Fermentation is the process of substrate-level phosphorylation, whereby ATP is derived from internally produced electron acceptors without a chemisomotic gradient.
Both processes are functionally related to maintain REDOX balance.
Whereas respiration involving burning of oxygen leads to profound heat production, fermentation is less effective due to lower enthropy.
In humans, in internal cell respiration, electrons are transferred from reduced Carbon substrates to the final electron acceptor oxygen.
Aerobic fermentation can proceed in the presence of oxygen. Negative effects of fermentation are acidification and energy deprivation.
Oxic and anoxic are terms to describe oxygen conditions in an environment.
Aerobic and anaerobic are terms used to refer to cell metabolism.
Chemisomotic coupling is one of the earliest events in single cells, i.e. bacteria, in evolution.
All single cells have a membrane-bound ATP-synthase.
ATP is produced by using gradients, whereby a chemisomotic potential is generated by Na- and proton pumps.
Protons are expelled into the cytosol, then move back into the intermembrane space. ATP is pulled out by ANT.
LEAK vs. SLIP hypothesis
Electron transport can be partially uncoupled, loosely coupled, dyscoupled or uncoupled. Protons are pumped out of the matrix and outside compartment. Where is the LEAK and how does it take place against a gradient? Different hypotheses exist on the nature vs. the quantification of LEAK. Is it dependent on oxygen flux?
Whereas LEAK seems to be a property of the membrane, SLIP seems to be a property of the pump, continuing at high ATP concentrations.
Temperature occurs to influence these properties, as membrane fluidity is affected. Experiments at 25°C favor SLIP - experiments at 37°C favor LEAK.
Sports reserach experiments are done at 22°C, although they rather should be done at 40°C implying high heat production inside myocytes during exercise.
Coupling states
Understanding and evaluation of coupling states can give you important information on e.g. organ or species differences, tissue injuries,...
CI, CII and CIV are pumps that pump protons out; uncouplers such as FCCP are protonophores, making protons flow back so that membrane potential eventually collapses. Every uncoupler also inhibits respiration at a certain concentration.
Substrates are actively transported across the mitochondrial membrane needing a gradient. Yeast mitochondria can also utilize cytosolic NADH, which is also produced in the TCA cycle, feeding electrons into the Q-junction via CI.
CI, CII & CIV as well as CII, CIII & CIV are referred to as "membrane-bound Electron Transfer System" ETS.
To find out what is limiting flux, you first have to take away any other limitations, e.g. substrates.
If submitochondrial particles are generated via e.g. sonication, the membrane structure gets disrupted and aligns spontaneously, possibly resulting in an inverted alignment, where NADH can be accessed from the outside, leading to a much larger ETS capacity.
Using the O2k, always the total ETS, incl. added substrates and transporters, is evaluated, which has to be differentiated from the membrane-bound ETS (pumps) that is mentioned in textbooks.
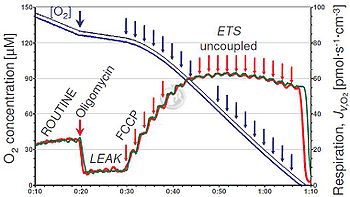
When looking at a coupling control protocol in intact cells Gnaiger 2008 POS, at first, without any manipulation, cells respire according to their routine ATP demand - ROUTINE.
By adding oligomycin, an inhibitor of ATP-synthase, a LEAK state is induced, as phosphorylation is eliminated. ATPase has 2 rotors. ATP has to be actively transported outside from the matrix space via AdenineNucleotideTranslocase ANT. ATP has a higher charge than ADP, and thus needs an electric gradient for transport.
Titrations of FCCP lead to an increase in respiration, reach a plateau (which is then marked as ETS, i.e. max. respiration) and then decrease respiration, due to inhibition. At ETS max. respiration, the high proton gradient does not have any feedback control on respiration. Titrations of FCCP show the dynamics of respiration, giving rise to uncoupler kinetics. Ethanol-soluble substances, such as FCCP, can penetrate membranes. As more oxygen is dissolved in EtOH, O2 concentrations might slightly increase after FCCP titrations. Also oligomycin is dissolved in EtOH and can pass through cell and mitochondrial membrane.
By titration of ADP, OXPHOS capacity can be maximized. The proton gradient is utilized to phosphorylate ADP. To evaluate OXPHOS capacity, one needs saturating ADP concentrations, which in vivo may or may not occur. When ADP is saturating, no flux increase occurs.
ATPase can also be added experimentally, which then regenerates ADP, thereby imitating a ROUTINE state.
Adding an uncoupler in an ADP-stimulated maximum OXPHOS state, can tell you about the limitation of OXPHOS.
If the "PHOS capacity", i.e. the utilizator, is bigger than the ETS, i.e. the generator, then you are probably dealing with an experimental artifact, as this can not be the case.
The maximum P/E ratio can only be 1.
If it is smaller than one, then the phosphorylation system is limiting.
ETS is not influenced by uncoupler. A total higher OXPHOS capacity indicates injury.
OXPHOS is influenced by in-efficiency = leakiness
The ATPase driving phosphorylation needs protons, but e.g. through holes in the membrane protons can leak, which can also be artificially increased by addition of uncouplers. On the other hand, this allows you to test unknown substances and to check whether they act as uncouplers by increaseing LEAK.
The mitochondrial system is not a machine - there is no ideal coupling even in healthy states. Intrinsic leak is a property of mitochondria - there is always loose coupling. Due to membrane injury or pharmacological damage, the leakiness can increase, leading to a dysfunction.
If there is - theoretically - no LEAK or SLIP, then the membrane potential increases and due to equilibrium, there is no oxygen flux. Leakiness increases flux. In respirometry, leakiness always has to be evaluated relative to a reference state. If there is less LEAK, there is less respiration.
Biochemical threshhold
Recommended literature:
- Vaupel P, Mayer A (2010) Evidence against a mitochondrial dysfunction in cancer cells as a hallmark of malignant growth.
- Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg's contributions to current concepts of cancer metabolism. Nature Reviews Cancer 11: 325-337.
- Crabtree HG (1929) Observations on the carbohydrate metabolism of tumours. Biochem J 23: 536–545.
- Keilin D (1925) On cytochrome, a respiratory pigment, common to animals, yeast, and higher plants. Proc R Soc Lond B Biol Sci 98: 312-339.
Oxidative stress is oxygen dependent.
What is NORMOXIA?
Test tube: 20kPa
Lung: 200 µM O2 ~ air saturation
Cell/mitochondria: 3 - 5 µM O2
FIGURE
Intrinsic LEAK (red) can be pathologically increased versus ETS
Reserve capacity = if ETS is higher than OXPHOS (E > P) -> apparent excess capacity = It is not known what this e--transfer is needed for (e.g. Ca2+-pumps?) OR Insurance against a potential enzymatic injury
Threshhold: genotype-phenotype relationships in mitochondrial diseases: the biochemical threshhold is defined as the niveau (ETS vs. OXPHOS) at which a pathological effect starts. Effect of mutant load on activity of respiratory complexes is different in different tissues.
If one enzyme in a chain/system is defect, the whole pathway suffers. Organs with a high biochemical threshhold are more resistant against a genetic defect, e.g. neurons vs. liver.
e--transport involves Complexes I - IV plus ETF plus Glycerophosphatedehydrogenase -> downstream is the complex phosphorylation system.
CII-linked respiration involves 2 proton pumps (CII is NOT a pump) -> CIII + CIV
CI-linked respiration has 3 proton pumps: CI, CIII and CIV
--> different stoichiometry, thus CII produces less ATP - OXPHOS -> less saturation
Procedure for evaluation of control of Complex IV - Work in progress
- Procedure is carried out in uncoupled state with FCCP -> ETS-capacity
- CIII is inhibited with AntA
- Cyt-c is reduced with Ascorbate and TMPD (together to prevent autoxidation)
- KCN/Cyanide titration to inhibit Cytochrome-c-Oxidase (if Pyruvate is used -> use Azide for inhibition)
- 3x Km equals 50% respiration; 9x Km is 75% inhibition and 19 times Michaelis-Menten constant equals 95% inhibition; thus, increase concentration of inhibitor in small steps - then double -> we want to get a hyperbole function
Flux Control Ratio -> normalize to non-inhibited value
FIGURE
- If Flux Control Coefficient is close to zero -> far from threshhold.
- FCC tells you how strongly an enzyme controls the entire pathway
- If additive effect of CI + CII strong, then artificial e--gating (e.g. CI only) leads to strong limitation of CIV (seems to be in control)
CAVE: always bear in mind the temperature-dependent activation of enzymes, e.g.: Hyperthermia (febrile seizures ~ Epilepsy) vs. Hypothermia (Avalanche vs. Cryopreservation) >> to be discussed at Hypothermia-Network: Mitochondria in the Cold