Description
Permeabilized muscle fibers (pfi) are used as a mitochondrial preparation in respirometry to access mitochondrial function comparable to isolated mitochondria (imt). pfi are obtained by selectively permeabilizing the plasma membrane mechanically and chemically (saponin), for the exchange of soluble molecules between the cytosolic phase and external medium, without damaging the mt-membranes.
- » MitoPedia topic: Mitochondrial preparations
Abbreviation: pfi
Reference: Doerrier 2018 Methods Mol Biol; MiPNet14.14 PermeabilizedFiberPreparation
- » Keywords
High oxygen in permeabilized fibers versus oxygen limitation under hypoxia
Contributed by Gnaiger E 2012-05-26, edited 2016-08-17.
Respiratory capacity and substrate concentrations
- When measuring respiration in permeabilized fibers (pfi), the arguments for the appropriate experimental oxygen regime are quite simple: If you are interested in measuring mitochondrial respiratory capacity (OXPHOS, Electron transfer pathway), substrate concentrations must be evaluated such that a limitation of respiratory flux by non-saturating substrate concentration can be excluded. Kinetic control studies, therefore, provide the basis of critically examining the appropriate substrate concentration for carbon substrates, ADP, inorganic phosphate and oxygen. Even if ADP concentrations in muscle tissues never reach 5 mM in vivo, one has to increase the ADP concentration to such high or even higher values in pfi, if the kinetics tells us that the more physiological 100 µM concentration does not support maximum respiratory flux.
- The same argument applies for oxygen supply. 200 µM (20 kPa) is far higher than physiological intracellular pO2 in muscle tissue. Nevertheless, isolated mitochondria are most frequently studied in the range of 10 to 20 kPa, rather than at physiological 0.5 to 3 kPa. This is fine as long as a concentration-independent saturating concentration range is obtained, where high substrate concentrations are neither inhibiting nor damaging. Air-level oxygen pressure, however, is effectively hyperoxic in isolated mitochondria (Gnaiger et al 2000), but may be hypoxic in permeabilized muscle fibers.
Oxygen kinetics of permeabilized fibers
- Unfortunately, the oxygen dependence of respiration in permeabilized fibers is about 100-fold higher compared to small living cells and isolated mitochondria (reviewed by Scandurra and Gnaiger 2010) due to artificial diffusion gradients resulting from the spatial constraints (long diffusion distances) in the fiber preparation (Gnaiger 2003, Pesta and Gnaiger 2012). When incubating pfi below air-level oxygen pressures at a physiological temperature of 37 °C, therefore, it must be shown that the preparation is not oxygen limited due to the spatial configuration of fiber bundles and development of a hypoxic core. Control experiments should be shown (rather than neglecting the problem of oxygen limitation) with traces of JO2 in the O2k over prolonged periods of time, when oxygen drops from air level to the minimum experimental values, which would provide proof that oxygen flux is stable and not progressively limited by the declining oxygen concentration. In pfi from various species and muscle types (including human, rat, mouse and fish heart; human, horse, rat, mouse, and fish skeletal muscle), oxygen limitation has been shown for pfi respiration below air level pO2. Undefined hypoxic limitation of respiration of a core population of mitochondria is not a satisfactory condition, hence hyperoxic incubation presents the best compromise for respiratory studies with pfi. Unfortunately, oxygen limitation of pfi has been ignored by several research groups (Kuznetsov et al 2008; compare Kuznetsov et al 1998; for a discussion see Pesta and Gnaiger 2012).
- Kuznetsov AV, Lassnig B, Margreiter R, Gnaiger E (1998) Diffusion limitation of oxygen versus ADP in permeabilized muscle fibers. In BioThermoKinetics in the Post Genomic Era (Larsson C, Påhlman I-L, Gustafsson L, eds) Chalmers Reproservice, Göteborg:273-6. - Bioblast link
- Gnaiger E, Méndez G, Hand SC (2000) High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci U S A 97:11080-5. - Bioblast link
- Gnaiger E (2003) Oxygen conformance of cellular respiration. A perspective of mitochondrial physiology. Adv Exp Med Biol 543:39-55. - Bioblast link
- Scandurra FM, Gnaiger E (2010) Cell respiration under hypoxia: Facts and artefacts in mitochondrial oxygen kinetics. Adv Exp Med Biol 662:7-25. - Bioblast link
- Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25-58. - Bioblast link
Oxygen dependence of ROS production - are permeabilized fibers a valid model?
- When studying mitochondrial ROS production, oxygen levels have to be controlled as a critical factor involved in hypoxic or hyperoxic stress. Considering uncontrolled oxygen gradients in permeabilized fibers, can this mitochondrial preparation provide a valid model for the study of mt-ROS production and ROS dynamics?
- The topic for uncontrolled oxygen concentration gradients in pfi, therefore, is becoming an even more disturbing issue when studying not only oxygen consumption but also ROS production. Should then oxygen levels be reduced to the more physiological range when working with pfi? In pfi, ROS production may be artificially increased by application of high oxygen pressures prevailing around a peripheral mt-subpopulation. On the other hand, ROS may be reduced in a central hypoxic subpopulation, and again increased if reductive stress increases ROS production at some intermediary pO2 levels along the oxygen gradient.
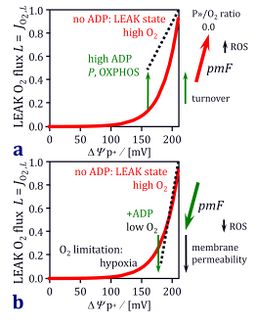
- We have tested the oxygen dependence of H2O2 production in a preparation of cardiac mitochondria during the O2k-Fluorometry Workshop IOC66. For illustration and discussion, we used a classical simple substrate (succinate&rotenone or the non-physiological condition of succinate only), and showed quantitatively the pronounced oxygen dependence of H2O2 production in the high oxygen range when mitochondrial respiration is fully oxygen-saturated (Boveris 1977[1]). An illustration of the complex interactions of LEAK respiration, membrane potential, hyperoxia and hypoxia is given in the figure (modified from Gnaiger 2001; for the figure legend, click on the figure).
- Gnaiger E (2001) Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir Physiol 128:277-97. - Bioblast link
- We have tested the oxygen dependence of H2O2 production in a preparation of cardiac mitochondria during the O2k-Fluorometry Workshop IOC66. For illustration and discussion, we used a classical simple substrate (succinate&rotenone or the non-physiological condition of succinate only), and showed quantitatively the pronounced oxygen dependence of H2O2 production in the high oxygen range when mitochondrial respiration is fully oxygen-saturated (Boveris 1977[1]). An illustration of the complex interactions of LEAK respiration, membrane potential, hyperoxia and hypoxia is given in the figure (modified from Gnaiger 2001; for the figure legend, click on the figure).
Bioblast alert 2012(03) / Talk:Permeabilized muscle fibers
Tissue homogenate versus permeabilized fibers
- The conclusion at this stage is that pfi may not provide an optimum model for studies of ROS production.
- On the other hand, isolated mitochondria (imt) require too much tissue for most of our studies on biopsies and on mice. To address these problems, we initiated a project on an alternative approach, using a high-quality mt-preparation with a high mt-yield. To achieve this goal, we tested the O2k-Set for muscle tissue homogenate preparation, not using any proteases (such as nagarse) nor saponin or digitonin, with merely 15 seconds of mechanical treatment, and completing the entire preparation within 10 min. We optimized the tissue homogenate (thom) preparation to obtain a 100% yield of functionally intact mitochondria. Respiration of muscle thom is less oxygen dependent compared to pfi, but some extent of hyperoxia remains necessary compared to imt to avoid any oxygen limitation.
- It is possible to obtain a single thom preparation with 2-4 mg of tissue, but also increase the amount of tissue to 50 mg in a single preparation, which then yields a thom that can be partitioned into several O2k-chambers for technical repeats or application of different protocols, and subsamples can be stored away for other tests. These tests convinced us, such that we are now offering the PBI-Shredder world-wide as an ‘Auxiliary O2k-Tool’.
- * PBI-Shredder O2k-Set: Preparation of tissue homogenates for diagnosis of mitochondrial respiratory function. Mitochondr Physiol Network 17.02. - Bioblast link
- A summary of test experiments (basic respirometry only) and a description of the shredder homogenization procedure is available.
- Mitochondrial respiration in permeabilized fibers versus homogenate from trout heart and liver. MiPNet17.03.
- A summary of test experiments (basic respirometry only) and a description of the shredder homogenization procedure is available.
- A demo experiment on respiration and hydrogen peroxide production of mouse heart homogenate with the [1] is shown in the workshop programme (download pdf file from IOC66).
Add to the discussion: Talk:Permeabilized muscle fibers
Historical perspectives
- The skinned muscle fiber technique was originally introduced by Endo (1967), derived from the mechanically skinned skeletal muscle fiber preparation, first introduced by Natori (1954). It was further adapted and modified by Veksler et al (1987) for cardiac muscle fibers, applied by Letellier et al (1992) for the first time for functional diagnosis of mitochondrial myopathies, and is now well established particularly for skeletal muscle fibers (Saks et al 1998).
- Then as now, it was of great importance “to have the characters closely resembling those of the intact fiber” (Natori 1954). The idea was: “from a physiological point of view, it is desirable to study the contractile system in a state as close to the living state as possible” (Saida and Nonomura 1978).
- After removal of the tissue from the species, the procedure allows mechanical separation of muscle tissue in a relaxing solution. Thereafter, muscle tissue is incubated in saponin by gentle agitation. Saponin, a chemical permeabilization agent of plant origin, selectively attacks cholesterol-rich (plasma) membranes of cells or muscle fibers, leaving intracellular membrane structures (mitochondria and ER) as well as myofilaments and the cytoskeleton intact. Consequently, the plasma membrane barrier integrity is lost and the cytosol and all solutes are washed out and the composition of the intracellular milieu is quickly equilibrated with the incubation medium. The mitochondria (in situ) are then able to use various substrates within a given protocol.
- Plasma membrane permeabilization with digitonin or saponin yields effective wash-out of free cytosolic molecules including adenylates, substrates, and cytosolic enzymes, making externally added compounds accessible to the mitochondria. ADP kinetics is fundamentally different in pfi and imt (Saks et al 1988). In addition, oxygen kinetics is shifted by a 100-fold increase of the p50 in pfi versus imt (Pesta and Gnaiger 2012).
- References
- Natori H (1954) The property and contraction process of isolated myofibrils. Jikei Med J 1
- Saida K, Nonomura Y (1978) Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol 72.
- Endo M (1967) Regulation of contraction relaxation of cycle (in japanese). Gen Assoc Jpn Med Congr Proc. 17:193-7.
- Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA (1987) Mitochondrial respiratory parameters in cardiac tissue: A novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta 892:191-6. - Bioblast link
- Letellier T, Malgat M, Coquet M, Moretto B, Parrot-Roulaud F, Mazat JP (1992) Mitochondrial myopathy studies on permeabilized muscle fibers. Pediatr Res 32:17–22. - Bioblast link
- Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS (1998) Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 184:81-100. - Bioblast link
- Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25-58. - Bioblast link
- References
Experimental SOP
Addition of fibers to the O2k-chamber
- While preparing the permeabilized fibers, perform oxygen calibration or the instrumental O2 background test.
- It is recommended to have an oxygen concentration of 450 µM in the O2k-chambers prior to the addition of the permeabilized muscle fibers.
- Immediately after wet mass determination, quickly open the O2k-chamber, insert the fiber preparation and close the O2k chamber. The oxygen level must be c. 400 µM. If it is necessary, increase oxygen concentration by (1) opening the chamber (lift the stopper to the stopper spacer position applying the Stopper-Spacer), inject oxygen, and close the chamber when the oxygen level reaches c. 400 µM; (2) injecting 1-3 µL H2O2 to the respiration medium in the presence of catalase (medium MiR06). See our O2k-Videosupport for fiber permabilization.
- Restart DatLab to define time zero as the time when fibers have been added.
- Immediately add P or G or Oct or Pal or .., then add M. Do not leave fibers without substrates. Do not add M first, which may lead to oxaloacetate accumulation, and prolong the establishment of a steady-state after the addition of P or G or Oct or Pal. A counter-argument might be (as a basis of our published protocols): In the initial phase without added substrates, endogenous substrates are oxidized preferentially when compared to a state with immediate addition of substrates. The prolonged LEAK state (rather than initial substrate-depleted state) proposed here in the alternative initial phase of the experiment will provide a prolonged evaluation of stabilization of flux (not disturbed by substrate additions), but stress due to substrate limitation may be worse than stress due to high membrane potential.
Increasing O2 concentrations
- To decrease or increase the oxygen concentration in the O2k-chamber, see Setting the oxygen concentration.
Chemicals and media
- What is the optimum ADP concentration to evaluate OXPHOS capacity in permeabilized muscle fibers?
- In muscle fibers, an ADP concentration of 5 mM or higher is needed in the kinetically-saturating range. In order to test if the applied ADP concentration is saturating, an additional ADP titration might be required.
- What is the optimum ADP concentration to evaluate OXPHOS capacity in permeabilized muscle fibers?
- » Pesta 2012 Methods Mol Biol: At a high apparent Km for ADP of 0.5 mM, flux at 2.5 and 5 mM ADP is ADP-limited by 13 % and 7 % (assuming L/P=0.2). 2.5 mM ADP is saturating in many cases, yet a further increase of ADP concentration provides a test for saturating [ADP]. This is particularly important for evaluation of OXPHOS versus ET capacity (P versus E or P/E coupling control ratio).
Permeabilized fibers: auxiliary O2k-tools
- Auxiliary O2k-tools are offered for fiber preparation (O2k-Dissection Set) and weight determination (Microbalance).
Tissue storage
- Muscle biopsies of horses can be stored for several days without loss of function[6]).
- Storage time without loss of function is, therefore, tissue and species-dependent and should be evaluated experimentally. When a larger tissue sample is available, separate the sample into small (10 mg) pieces, and apply respirometric SUIT protocols on subsamples in a time course. In particular, evaluate dyscoupling (L/E and P/E coupling control ratios), cytochrome c release,[7] and OXPHOS capacities with various substrate combinations.
References
| Bioblast link | Reference | Year |
|---|---|---|
| BEC 2020.1 doi10.26124bec2020-0001.v1 | Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1 | 2020 |
- ↑ Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol. 1977;78:67-82. doi: 10.1007/978-1-4615-9035-4_5. PMID: 197811.
- ↑ Gnaiger E, Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Steurer W, Margreiter R (2000) Mitochondria in the cold. In: Life in the Cold (Heldmaier G, Klingenspor M, eds) Springer, Heidelberg, Berlin, New York:431-42. - Bioblast link
- ↑ Kuznetsov AV, Strobl D, Ruttmann E, Königsrainer A, Margreiter R, Gnaiger E (2002) Evaluation of mitochondrial respiratory function in small biopsies of liver. Analyt Biochem 305:186-94. - Bioblast link
- ↑ Skladal D, Sperl W, Schranzhofer R, Krismer M, Gnaiger E, Margreiter R, Gellerich FN (1994) Preservation of mitochondrial functions in human skeletal muscle during storage in high energy preservation solution (HEPS). In: What is Controlling Life? (Gnaiger E, Gellerich FN, Wyss M, eds) Modern Trends in BioThermoKinetics 3. Innsbruck Univ Press:268-71 - Bioblast link
- ↑ Lemieux H, Semsroth S, Antretter H, Hoefer D, Gnaiger E (2011) Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol 43:1729–38. - Bioblast link
- ↑ Votion DM, Gnaiger E, Lemieux H, Mouithys-Mickalad A, Serteyn D (2012) Physical fitness and mitochondrial respiratory capacity in horse skeletal muscle. PLoS One 7: e34890. - Bioblast link
- ↑ Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Mark W, Steurer W, Saks V, Usson Y, Margreiter R, Gnaiger E (2004) Mitochondrial defects and heterogeneous cytochrome c release after cardiac cold ischemia and reperfusion. Am J Physiol Heart Circ Physiol 286:H1633–41. - Bioblast link
- The following references describe in detail the basis for working with muscle fibers in the O2k.
- Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25-58. - Bioblast link
- Lemieux H, Semsroth S, Antretter H, Hoefer D, Gnaiger E (2011) Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol 43:1729–38. - Bioblast link
- Votion DM, Gnaiger E, Lemieux H, Mouithys-Mickalad A, Serteyn D (2012) Physical fitness and mitochondrial respiratory capacity in horse skeletal muscle. PLoS One 7: e34890. - Bioblast link
- Preparation of permeabilized muscle fibers for diagnosis of mitochondrial respiratory function. Mitochondr Physiol Network 14.14. - Bioblast link
- Isolated mitochondria or permeabilized tissues and cells. Mitochondr Physiol Network 11.05. - Bioblast link
- Mitochondrial respiration medium - MiR06. Mitochondr Physiol Network 14.13. - Bioblast link
- MitoPedia topic: Permeabilized tissue or cells.
MitoPedia topics:
Sample preparation