Bertero 2022 Function (Oxf)
| Bertero E, Maack C (2022) Rethinking Mitchell's chemiosmotic theory: potassium dominates over proton flux to drive mitochondrial F1Fo-ATP synthase. Function (Oxf) 3:zqac012. https://doi.org/10.1093/function/zqac012 |
Bertero E, Maack C (2022) Function (Oxf)
Abstract: In the current issue of Function, Juhaszova and colleagues3 substantially challenge—or rather expand, but do not tumble—this concept in revealing that in addition to H+, potassium ion (K+) flux through the F1Fo-ATP synthase (working the same way as H+) provides the majority of energy to produce ATP (Figure 1). Why was this was overlooked for more than six decades? Presumably because the F1Fo-ATP synthase has a > 107-fold selectivity for H+ over other cations.4 But what was not sufficiently considered is that due to the 106-fold higher cytosolic concentration for K+ (∼100 mM) than for H+(∼100 nM), such that K+ flux—driven mostly by the same high electrical driving force (∆Ψm) - could be comparable to H+ flux via the ATP synthase.
• Bioblast editor: Gnaiger E
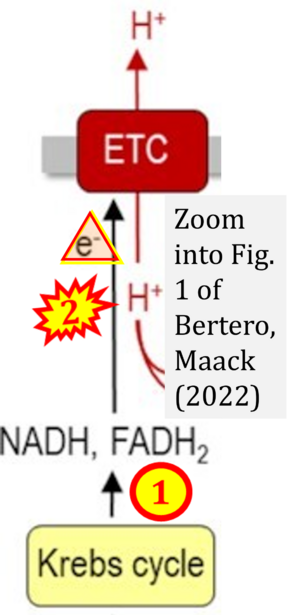
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex V;ATP synthase Regulation: ATP production, Ion;substrate transport, mt-Membrane potential