Blanco 2017 Academic Press
| Blanco A, Blanco G (2017) Chapter 9 - Biological oxidations: bioenergetics. In Blanco A, Blanco G, eds, Medical biochemistry. Academic Press:177-204. https://doi.org/10.1016/B978-0-12-803550-4.00009-4 |
» https://doi.org/10.1016/B978-0-12-803550-4.00009-4
Blanco A, Blanco G (2017) Academic Press
Abstract: Biological oxidations do not take place by direct transfer of electrons (e−) from substrate to oxygen. They are carried out in successive stages by different e− acceptors with increasing reduction potential. This allows for a stepwise release of energy and its best utilization by the cell. The respiratory chain, located in the mitochondrial inner membrane, comprises a series of H or electrons (e−) acceptors arranged according to increasing reduction potential, associated with enzymes that catalyze e− transfer. It is composed of complex I or NADH–ubiquinone reductase, complex II or succinate–ubiquinone reductase, ubiquinone or coenzyme Q, complex III or ubiquinone–cytochrome c reductase, cytochrome c, located on the outer face of inner membrane, complex IV or cytochrome oxidase. Finally 4 e− are transferred to 2 O atoms, which with 4 H+ form 2 H2O. The energy produced by the flow of e− is coupled to phosphoryl transfer, synthesizing adenosine triphosphate (ATP) from ADP in a process known as oxidative phosphorylation. Each e− pair from substrates of NAD-linked dehydrogenases generates three molecules of ATP, while substrates oxidized by FAD-dependent enzymes produce two ATP. The chemio-osmotic hypothesis explains the mechanism underlying oxidative phosphorylation. The energy generated by the flow of reducing equivalents is used to pump protons from the mitochondrial matrix outward into the inner membrane, at the site of complexes I, III, and IV. The proton gradient created across the mitochondrial inner membrane drives proton flux through the F1F0 or ATP synthase complex, which couples proton transport to phosphate addition to ADP. Compounds that reduce or eliminate the proton gradient inhibit phosphorylation. Inhibitors can block e− transfer at different levels of the respiratory chain. Rotenone, amytal, and other barbiturates act at the level of complex I; antimycin A, at complex III; and cyanide, carbon monoxide, and azide, on complex IV. Inhibitors of oxidative phosphorylation include proton and K+ ionophores, which suppress the mitochondrial electrical potential gradient, acting as uncoupling agents, and compounds, such as oligomycin, which interfere with the function of the F1F0 ATPase. Brown fat present in infants and hibernating animals has thermogenin, a protein that inhibits ATP synthesis, uncoupling mitocondrial function and contributing to maintain body temperature. Oxidative phosphorylation is mainly controlled by ADP levels. Phosphorylation at substrate level is another way to generate ATP by phosphoryl transfer from high energy metabolites.
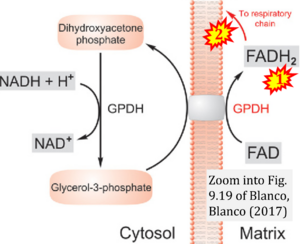
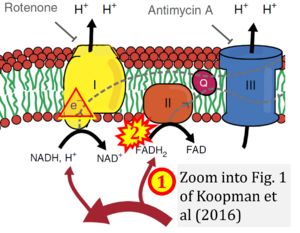
- Comment (Cardoso Luiza, Gnaiger Erich, 2023-08-05): Figure 9.19 shows FADH2 to be formed in the mitochondrial matrix from (1) GPDH (CGpDH), feeding electrons further (2) "To respiratory chain'. Combined with FADH2 formed from (1) the TCA cycle and feeding (2) into CII (Fig. 1 on the right; one of >120 examples discussed as CII-ambiguities), one may arrive at the erroneous conclusion on a direct role of CII in the oxidation of glycerol-3-phosphate, analogous to false representations of CII involved in fatty acid oxidation.
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: