Cerqua 2021 Springer, Cham
| Cerqua C, Buson L, Trevisson E (2021) Mutations in assembly dactors required for the biogenesis of mitochondrial respiratory chain. In: Navas P, Salviati L (eds) Mitochondrial diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-70147-5_2. |
Cerqua C, Buson L, Trevisson E (2021) Springer, Cham
Abstract: The mitochondrial respiratory chain, which provides to the cells most of their ATP requirement, is composed of five multisubunit complexes. Its biogenesis is a multi-step process characterized by the sequential formation of intermediate assemblies composed of subunits encoded by two distinct genomes, mitochondrial or nuclear DNA.
This process is assisted by a diverse set of ancillary proteins of nuclear origin called assembly factors that are not part of the final complexes and exert different functions.
Mutations in several genes encoding these proteins have been identified in patients affected by mitochondrial diseases, exceeding those found in genes encoding structural subunits for some complexes.
The hallmark of these disorders, which are often multisystemic and mainly affect high energy demanding organs, is the broad genetic and clinical heterogeneity, making their diagnosis problematic. The number of assembly factors associated with human diseases is rapidly increasing, owing to the employment of next generation sequencing methods in the diagnostic workflow.
Therapy for these conditions is mostly based on supportive care, emphasizing the need to elucidate their pathological mechanisms to find novel treatments.
• Bioblast editor: Gnaiger E
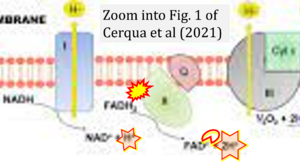
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.