Fischer 2021 Antioxidants
| Fischer C, Volani C, Komlódi T, Seifert M, Demetz E, Valente de Souza L, Auer K, Petzer V, von Raffay L, Moser P, Gnaiger E, Weiss G (2021) Dietary iron overload and Hfe-/- related hemochromatosis alter hepatic mitochondrial function. https://doi.org/10.3390/antiox10111818 |
» Antioxidants 10:1818. MDPI Open Access
Fischer Christine, Volani Chiara, Komlodi Timea, Seifert Markus, Demetz Egon, Valente de Souza Lara, Auer Kristina, Petzer Verena, von Raffay Laura, Moser Patrizia, Gnaiger Erich, Weiss Guenter (2021) Antioxidants
Abstract:
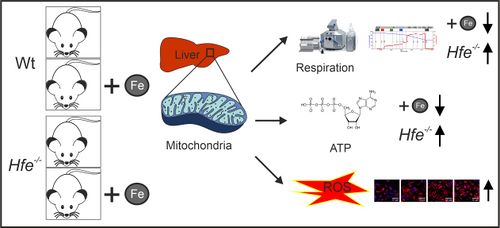
Iron is an essential co-factor for many cellular metabolic processes, and mitochondria are main sites of utilization. Iron accumulation promotes production of reactive oxygen species (ROS) via the catalytic activity of iron species. Herein, we investigated the consequences of dietary and genetic iron overload on mitochondrial function. C57/BL6N wildtype and Hfe-/- mice, the latter a genetic hemochromatosis model, received either normal diet (ND) or high iron diet (HI) for two weeks. Liver mitochondrial respiration was measured using high-resolution respirometry along with analysis of expression of specific proteins and ROS production. HI promoted tissue iron accumulation and slightly affected mitochondrial function in wildtype mice. Hepatic mitochondrial function was impaired in Hfe-/- mice on ND and HI. Compared to wildtype mice, Hfe-/- mice on ND showed increased mitochondrial respiratory capacity. Hfe-/- mice on HI showed very high liver iron levels, decreased mitochondrial respiratory capacity and increased ROS production associated with reduced mitochondrial aconitase activity. Although Hfe-/- resulted in increased mitochondrial iron loading, the concentration of metabolically reactive cytoplasmic iron and mitochondrial density remained unchanged. Our data shows multiple effects of dietary and genetic iron loading on mitochondrial function and linked metabolic pathways, providing an explanation for fatigue in iron-overloaded hemochromatosis patients and suggests iron reduction therapy for improvement of mitochondrial function.
• Keywords: hemochromatosis; iron overload; reactive oxygen species, ROS; mitochondria, mt; mitochondrial respiration; liver; fatigue
• Bioblast editor: Gnaiger E
• O2k-Network Lab: AT Innsbruck Oroboros
ORCID: ![]() Fischer C,
Fischer C, ![]() Volani C,
Volani C, ![]() Komlodi T, Seifert M, Demetz E,
Komlodi T, Seifert M, Demetz E, ![]() Valente de Souza L, Auer K, Petzer V, von Raffay L, Moser P,
Valente de Souza L, Auer K, Petzer V, von Raffay L, Moser P, ![]() Gnaiger E,
Gnaiger E, ![]() Weiss G
Weiss G
Article processing charge
- MDPI: € 1623.08
- "Assuming a publication charge of € 1200 per article, scientists mentioning mitochondria or photosynthesis pay € 100 000 every day in 2021 for ‘selling’ their output to publishers ― over € 36 Mill per year."
- - Gnaiger E (2021) Beyond counting papers – a mission and vision for scientific publication. https://doi.org/10.26124/bec:2021-0005
MitoFit Preprints
- Fischer C, Volani C, Komlódi T, Seifert M, Demetz E, Valente de Souza L, Auer K, Petzer V, von Raffay L, Moser P, Gnaiger E, Weiss G (2021) Dietary iron overload and Hfe-/- related hemochromatosis alter hepatic mitochondrial function. MitoFit Preprints 2021.9. doi:10.26124/mitofit:2021-0009
Labels: MiParea: Respiration
Organism: Mouse
Tissue;cell: Liver
Coupling state: LEAK, OXPHOS, ET Pathway: ROX, N, S, Gp HRR: Oxygraph-2k