Garrido-Perez 2020 Int J Mol Sci
| Garrido-Pérez N, Vela-Sebastián A, López-Gallardo E, Emperador S, Iglesias E, Meade P, Jiménez-Mallebrera C, Montoya J, Bayona-Bafaluy MP, Ruiz-Pesini E (2020) Oxidative phosphorylation dysfunction modifies the cell secretome. Int J Mol Sci 21:3374. https://doi.org/10.3390/ijms21093374 |
Garrido-Perez N, Vela-Sebastian A, Lopez-Gallardo E, Emperador S, Iglesias E, Meade P, Jimenez-Mallebrera C, Montoya J, Bayona-Bafaluy MP, Ruiz-Pesini E (2020) Int J Mol Sci
Abstract: Mitochondrial oxidative phosphorylation disorders are extremely heterogeneous conditions. Their clinical and genetic variability makes the identification of reliable and specific biomarkers very challenging. Until now, only a few studies have focused on the effect of a defective oxidative phosphorylation functioning on the cell's secretome, although it could be a promising approach for the identification and pre-selection of potential circulating biomarkers for mitochondrial diseases. Here, we review the insights obtained from secretome studies with regard to oxidative phosphorylation dysfunction, and the biomarkers that appear, so far, to be promising to identify mitochondrial diseases. We propose two new biomarkers to be taken into account in future diagnostic trials.
• Bioblast editor: Gnaiger E
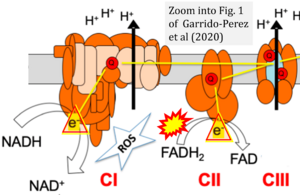
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.