Nakane 2020 J Intensive Care
| Nakane M (2020) Biological effects of the oxygen molecule in critically ill patients. J Intensive Care 8:95. https://doi.org/10.1186/s40560-020-00505-9 |
Nakane M (2020) J Intensive Care
Abstract: The medical use of oxygen has been widely and frequently proposed for patients, especially those under critical care; however, its benefit and drawbacks remain controversial for certain conditions. The induction of oxygen therapy is commonly considered for either treating or preventing hypoxia. Therefore, the concept of different types of hypoxia should be understood, particularly in terms of their mechanism, as the effect of oxygen therapy principally varies by the physiological characteristics of hypoxia. Oxygen molecules must be constantly delivered to all cells throughout the human body and utilized effectively in the process of mitochondrial oxidative phosphorylation, which is necessary for generating energy through the formation of adenosine triphosphate. If the oxygen availability at the cellular level is inadequate for sustaining the metabolism, the condition of hypoxia which is characterized as heterogeneity in tissue oxygen tension may develop, which is called dysoxia, a more physiological concept that is related to hypoxia. In such hypoxic patients, repetitive measurements of the lactate level in blood are generally recommended in order to select the adequate therapeutic strategy targeting a reduction in lactate production. Excessive oxygen, however, may actually induce a hyperoxic condition which thus can lead to harmful oxidative stress by increasing the production of reactive oxygen species, possibly resulting in cellular dysfunction or death. In contrast, the human body has several oxygen-sensing mechanisms for preventing both hypoxia and hyperoxia that are employed to ensure a proper balance between the oxygen supply and demand and prevent organs and cells from suffering hyperoxia-induced oxidative stress. Thus, while the concept of hyperoxia is known to have possible adverse effects on the lung, the heart, the brain, or other organs in various pathological conditions of critically ill patients, and no obvious evidence has yet been proposed to totally support liberal oxygen supplementation in any subset of critically ill patients, relatively conservative oxygen therapy with cautious monitoring appears to be safe and may improve the outcome by preventing harmful oxidative stress resulting from excessive oxygen administration. Given the biological effects of oxygen molecules, although the optimal target levels remain controversial, unnecessary oxygen administration should be avoided, and exposure to hyperoxemia should be minimized in critically ill patients.
• Bioblast editor: Gnaiger E
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
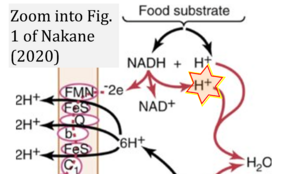
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
- Fig. 1 (Nakane 2020) is copied with permission from: Hall J (2016) Guyton and Hall Textbook of Medical Physiology. 13. Elsevier, Philadelphia. In H+-linked electron transfer, the 2e- should be matched with 2H+.
Labels:
Stress:Hypoxia