Paprocka 2022 Metabolites
| Paprocka J, Nowak M, Chuchra P, Śmigiel R (2022) COQ8A-ataxia as a manifestation of primary coenzyme Q deficiency. Metabolites 12:955. https://doi.org/10.3390/metabo12100955 |
Paprocka J, Nowak M, Chuchra P, Smigiel R (2022) Metabolites
Abstract: COQ8A-ataxia is a mitochondrial disease in which a defect in coenzyme Q10 synthesis leads to dysfunction of the respiratory chain. The disease is usually present as childhood-onset progressive ataxia with developmental regression and cerebellar atrophy. However, due to variable phenotype, it may be hard to distinguish from other mitochondrial diseases and a wide spectrum of childhood-onset cerebellar ataxia. COQ8A-ataxia is a potentially treatable condition with the supplementation of coenzyme Q10 as a main therapy; however, even 50% may not respond to the treatment. In this study we review the clinical manifestation and management of COQ8A-ataxia, focusing on current knowledge of coenzyme Q10 supplementation and approach to further therapies. Moreover, the case of a 22-month-old girl with cerebellar ataxia and developmental regression will be presented.
• Bioblast editor: Gnaiger E
Labels:
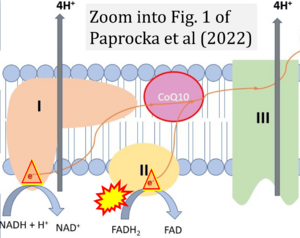
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.