Preston 2023 Academic Press
| Preston G, El Soufi El Sabbagh D, Emmerzaal TL, Morava E, Andreazza AC, Rahman S, Kozicz T (2023) Antidepressants, mood-stabilizing drugs, and mitochondrial functions: For better or for worse. In: de Oliveira MR (ed) Mitochondrial intoxication:323-49. Academic Press. https://doi.org/10.1016/B978-0-323-88462-4.00016-X |
Preston G, El Soufi El Sabbagh D, Emmerzaal TL, Morava E, Andreazza AC, Rahman S, Kozicz T (2023) Academic Press
Abstract: Psychiatric manifestations of primary mitochondrial diseases are increasingly recognized, and optimal strategies for managing these represent a significant unmet clinical need for this group of patients with complex multisystem symptomatology and high mortality and morbidity. Secondary mitochondrial dysfunction and oxidative stress have also been implicated in the pathophysiology of various psychiatric diseases. It has been well documented that medications used to treat neuropsychiatric disorders could directly modulate mitochondrial functions. The objective of this book chapter is to review antidepressant drugs and other psychiatric medications that are available for the treatment of psychiatric disorders and the evidence that exists in relation to the mitochondria using these drugs.
• Bioblast editor: Gnaiger E
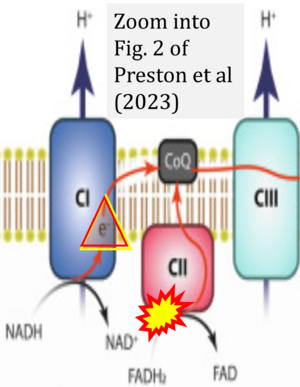
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.