Schniertshauer 2023 Curr Issues Mol Biol
| Schniertshauer D, Wespel S, Bergemann J (2023) Natural mitochondria targeting substances and their effect on cellular antioxidant system as a potential benefit in mitochondrial medicine for prevention and remediation of mitochondrial dysfunctions. Curr Issues Mol Biol 45:3911-32. https://doi.org/10.3390/cimb45050250 |
Schniertshauer D, Wespel S, Bergemann J (2023) Curr Issues Mol Biol
Abstract: Based on the knowledge that many diseases are caused by defects in the metabolism of the cells and, in particular, in defects of the mitochondria, mitochondrial medicine starts precisely at this point. This new form of therapy is used in numerous fields of human medicine and has become a central focus within the field of medicine in recent years. With this form of therapy, the disturbed cellular energy metabolism and an out-of-balance antioxidant system of the patient are to be influenced to a greater extent. The most important tool here is mitotropic substances, with the help of which attempts are made to compensate for existing dysfunction. In this article, both mitotropic substances and accompanying studies showing their efficacy are summarized. It appears that the action of many mitotropic substances is based on two important properties. First, on the property of acting antioxidantly, both directly as antioxidants and via activation of downstream enzymes and signaling pathways of the antioxidant system, and second, via enhanced transport of electrons and protons in the mitochondrial respiratory chain.
• Bioblast editor: Gnaiger E
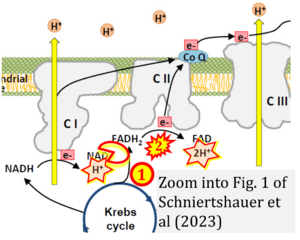
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels: MiParea: Pharmacology;toxicology