Steiner 2017 Int J Biochem Cell Biol
| Steiner JL, Lang CH (2017) Etiology of alcoholic cardiomyopathy: Mitochondria, oxidative stress and apoptosis. Int J Biochem Cell Biol 89:125-35. https://doi.org/10.1016/j.biocel.2017.06.009 |
Steiner JL, Lang CH (2017) Int J Biochem Cell Biol
Abstract: Putative mechanisms leading to the development of alcoholic cardiomyopathy (ACM) include the interrelated cellular processes of mitochondria metabolism, oxidative stress and apoptosis. As mitochondria fuel the constant energy demands of this continually contracting tissue, it is not surprising that alcohol-induced molecular changes in this organelle contribute to cardiac dysfunction and ACM. As the causal relationship of these processes with ACM has already been established, the primary objective of this review is to provide an update of the experimental findings to more completely understand the aforementioned mechanisms. Accordingly, recent data indicate that alcohol impairs mitochondria function assessed by membrane potential and respiratory chain activity. Indictors of oxidative stress including superoxide dismutase, glutathione metabolites and malondialdehyde are also adversely affected by alcohol oftentimes in a sex-dependent manner. Additionally, myocardial apoptosis is increased based on assessment of TUNEL staining and caspase activity. Recent work has also emerged linking alcohol-induced oxidative stress with apoptosis providing new insight on the codependence of these interrelated mechanisms in ACM. Attention is also given to methodological differences including the dose of alcohol, experimental model system and the use of males versus females to highlight inconsistencies and areas that would benefit from establishment of a consistent model.
• Bioblast editor: Gnaiger E
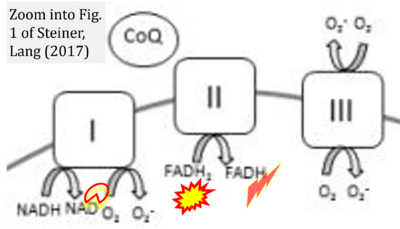
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels: MiParea: Pharmacology;toxicology
Stress:Oxidative stress;RONS
Tissue;cell: Heart
Enzyme: Complex II;succinate dehydrogenase
EtOH