Yang 2022 J Cleaner Production
| Yang Y, Zhang X, Hu X, Zhao J, Chen X, Wei X, Yu X (2022) Analysis of the differential metabolic pathway of cultured Chlorococcum humicola with hydroquinone toxic sludge extract. J Cleaner Production 370:133486. https://doi.org/10.1016/j.jclepro.2022.133486 |
Yang Yingying, Zhang Xinyu, Hu Xueyang, Zhao Jiamin, Chen Xiurong, Wei Xiao, Yu Xiao (2022) J Cleaner Production
Abstract: Microbe inhibition produces intermediate metabolites and toxic substances that accumulate significantly in residual sludge toxicity after continuous treatment of coal gasification wastewater with activated sludge. To address this issue, Chlorococcum humicola was cultured with sludge extract. Proteomics analysis was used to investigate the mechanism of stress response in microalgae to sludge toxicity. Upon completion of growth, microalgae treated with sludge extract produced 0.725 ± 0.036 g/L of biomass. The total organic carbon concentration and toxicity removal rates on microalgae were 75.1 ± 3.8% and 47.4 ± 2.1%, respectively. The proteomics analysis revealed that under stress conditions, microalgae altered cellular components such as the nucleus and plasma membranes, affecting the photosynthetic and respiratory processes. The logarithmic phase was characterized by a down-regulation of oxidative phosphorylation and an increase in energy synthesis regulated by the isopropyl phosphate isomerase. The results demonstrate a proteomic basis for the reduction and utilization of waste sludge, as well as provide a molecular mechanism reference for optimizing the model of cultivating microalgae using sludge.
• Bioblast editor: Gnaiger E
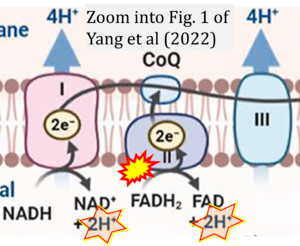
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.