Doerrier 2018 Methods Mol Biol
| Doerrier C, Garcia-Souza LF, Krumschnabel G, Wohlfarter Y, Mészáros AT, Gnaiger E (2018) High-Resolution FluoRespirometry and OXPHOS protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol Biol 1782:31-70. https://doi.org/10.1007/978-1-4939-7831-1_3 |
» PMID: 29850993 »![]()
Doerrier Carolina, Garcia-Souza Luiz F, Krumschnabel Gerhard, Wohlfarter Yvonne, Meszaros Andras T, Gnaiger Erich (2018) Methods Mol Biol
Abstract: Protocols for high-resolution respirometry of intact cells, permeabilized cells, permeabilized muscle fibers, isolated mitochondria and tissue homogenates offer sensitive diagnostic tests of integrated mitochondrial function using standard cell culture techniques, small needle biopsies of muscle, and mitochondrial preparation methods. Multiple substrate-uncoupler-inhibitor titration (SUIT) protocols for analysis of oxidative phosphorylation (OXPHOS) improve our understanding of mitochondrial respiratory control and the pathophysiology of mitochondrial diseases. Respiratory states are defined in functional terms to account for the network of metabolic interactions in complex SUIT protocols with stepwise modulation of coupling control and electron transfer pathway states. A regulated degree of intrinsic uncoupling is a hallmark of oxidative phosphorylation, whereas pathological and toxicological dyscoupling is evaluated as a mitochondrial defect. The noncoupled state of maximum respiration is experimentally induced by titration of established uncouplers (CCCP, FCCP, DNP), to collapse the protonmotive force across the mitochondrial inner membrane and measure the electron transfer capacity (ET; open-circuit operation of respiration). Intrinsic uncoupling and dyscoupling are evaluated as the flux control ratio between non-phosphorylating LEAK respiration (electron flow coupled to proton pumping to compensate for proton leaks) and ET capacity. If OXPHOS capacity (maximally ADP stimulated O2 flux) is less than ET capacity, the phosphorylation pathway contributes to flux control. Physiological substrate combinations supporting the NADH&succinate-pathway are required to reconstitute tricarboxylic acid cycle function. This supports maximum ET and OXPHOS capacities, due to the additive effect of multiple electron supply pathways converging at the Q-junction. ET-pathways with electron entry separately through NADH (pyruvate&malate or glutamate&malate) or succinate (succinate&rotenone) restricts ET capacity and artificially enhances flux control upstream of the Q-cycle, providing diagnostic information on specific ET-pathway branches. O2 concentration is maintained above air saturation in protocols with permeabilized muscle fibers to avoid experimental O2 limitation of respiration. Standardized two-point calibration of the polarographic oxygen sensor (static sensor calibration), calibration of the sensor response time (dynamic sensor calibration), and evaluation of instrumental background O2 flux (systemic flux compensation) provide the unique experimental basis for high accuracy of quantitative results and quality control in high-resolution respirometry.
• Bioblast editor: Gnaiger E • O2k-Network Lab: AT Innsbruck Gnaiger E, AT Innsbruck Oroboros
O2k-brief
RP1 and RP2 in mt-preparations
- SUIT RP1:1PM;2D:2c;3U;4G;5S;6Oct;7Rot;8Gp;9Ama;10AsTm;11Azd
- SUIT RP2:1D;2M.1;3Oct;3c;4M2;5P;6G;7S;8Gp;9U;10Rot;11Ama;12AsTm;13Azd
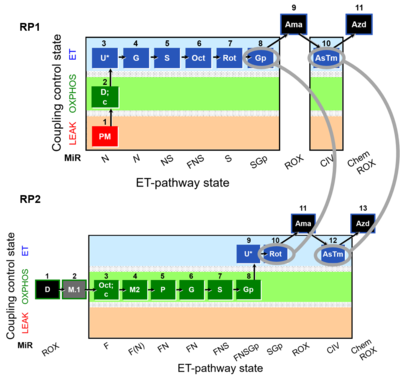 Harmonization between RP1 and RP2
Harmonization between RP1 and RP2
Correction
In Table 1, final concentration of oligomycin in a 2 mL O2k-chamber should be 2.5 µM instead of 2.5 µg/mL. Of note, it is mentioned in the text: “the use of 2.5 µM oligomycin may show an inhibitory effect on ET capacity in some biological samples (e.g. platelets)". Therefore, we recommend to 1) test the inhibitory effect of oligomycin on ET capacity (by uncoupler titrations in the absence of inhibitor) and, 2) evaluate lower oligomycin concentration (5-10 nM) to replace the standard 2.5 µM oligomycin concentration. See more details in oligomycin.
Cited by
- 37 articles in PubMed (2021-12-27) https://pubmed.ncbi.nlm.nih.gov/29850993/
- Gnaiger E (2021) Bioenergetic cluster analysis – mitochondrial respiratory control in human fibroblasts. MitoFit Preprints 2021.8. https://doi.org/10.26124/mitofit:2021-0008
- Krako Jakovljevic N, Ebanks B, Katyal G, Chakrabarti L, Markovic I, Moisoi N (2021) Mitochondrial homeostasis in cellular models of Parkinson’s Disease. Bioenerg Commun 2021.2. https://doi.org/10.26124/bec:2021-0002
- Komlódi T, Cardoso LHD, Doerrier C, Moore AL, Rich PR, Gnaiger E (2021) Coupling and pathway control of coenzyme Q redox state and respiration in isolated mitochondria. Bioenerg Commun 2021.3. https://doi.org/10.26124/bec:2021-0003
- Cardoso et al (2021) Magnesium Green for fluorometric measurement of ATP production does not interfere with mitochondrial respiration. Bioenerg Commun 2021.1. doi:10.26124/bec:2021-0001
- Went N, Di Marcello M, Gnaiger E (2021) Oxygen dependence of photosynthesis and light-enhanced dark respiration studied by High-Resolution PhotoRespirometry. MitoFit Prep 2021.5. - »Bioblast link«
- Komlódi T, Sobotka O, Gnaiger E (2021) Facts and artefacts on the oxygen dependence of hydrogen peroxide production using Amplex UltraRed. Bioenerg Commun 2021.4. https://doi:10.26124/BEC:2021-0004
- Komlódi T, Schmitt S, Zdrazilova L, Donnelly C, Zischka H, Gnaiger E. Oxygen dependence of hydrogen peroxide production in isolated mitochondria and permeabilized cells. MitoFit Preprints (in prep).
- Silva et al (2021) Off-target effect of etomoxir on mitochondrial Complex I. MitoFit Preprints 2021. (in preparation)
- Komlodi et al (2022) Hydrogen peroxide production, mitochondrial membrane potential and the coenzyme Q redox state measured at tissue normoxia and experimental hyperoxia in heart mitochondria. MitoFit Preprints 2021 (in prep)
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- Gnaiger E (2021) Bioenergetic cluster analysis – mitochondrial respiratory control in human fibroblasts. MitoFit Preprints 2021.8. https://doi.org/10.26124/mitofit:2021-0008
- Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1.
Labels: MiParea: Respiration, Instruments;methods
Organism: Human, Mouse, Rat, Saccharomyces cerevisiae
Tissue;cell: Heart, Skeletal muscle, Endothelial;epithelial;mesothelial cell, Blood cells, HEK, Platelet
Preparation: Permeabilized cells, Permeabilized tissue, Homogenate, Isolated mitochondria, Intact cells
Regulation: Oxygen kinetics Coupling state: LEAK, ROUTINE, OXPHOS, ET Pathway: F, N, S, Gp, CIV, NS, Other combinations, ROX HRR: Oxygraph-2k, TIP2k, O2k-Protocol
SUIT-001, SUIT-001 O2 mt D001, SUIT-001 O2 pfi D002, SUIT-001 O2 ce-pce D003, SUIT-001 O2 ce-pce D004, SUIT-002, SUIT-002 O2 mt D005, SUIT-002 O2 pfi D006, SUIT-002 O2 ce-pce D007, SUIT-002 O2 ce-pce D007a, SUIT-003 O2 ce-pce D020, SUIT-010, SUIT-010 O2 pce D008, SUIT-010, SUIT-010 O2 ce-pce D050, SUIT-010 O2 ce-pce D008, MitoPathways, MitoFitPublication, MitoEAGLEPublication, O2k-chemicals and media, MitoEAGLE blood cells reviews, O2k-brief, Flux control ratio, BEC 2020.1, BEC 2020.2, MitoFit 2021 MgG, MitoFit 2021 CoQ, MitoFit 2021.5 PB, MitoFit 2021 AmR-O2, MitoFit 2021 AmR, MitoFit 2021 Tissue normoxia, MitoFit 2022 NADH, MitoFit 2021 BCA, MitoFit 2021 PLT, PLoSONE2022ace-sce, MitoFit2022QC



