Description
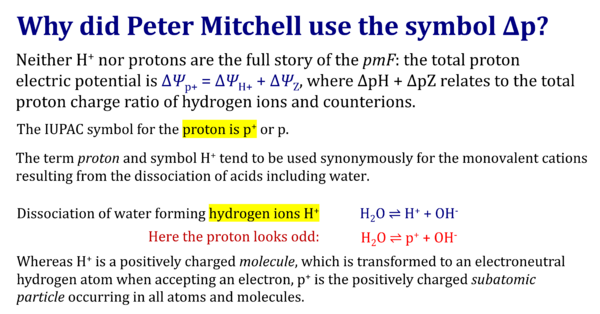
The terms hydrogen ion H+ and proton, p or p+, are used synonymously in chemistry. A hydrogen ion is a positively charged molecule. In particle physics, however, a proton is a submolecular and subatomic particle with a positive electric charge. The H+ ion has no electrons and is a bare charge with only about 1/64 000 of the radius of a hydrogen atom. Free H+ is extremely reactive, with an extremely short lifetime in aqueous solutions. There H+ forms the hydronium ion H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. The transfer of H+ in an acid–base reaction is referred to as proton transfer. The acid is the H+ donor and the base is the H+ acceptor.
Abbreviation: H+
Reference: Bunnett, Jones 1988, Headrick JM, Diken EG, Walters RS, Hammer NI, Christie RA, Cui J, Myshakin EM, Duncan MA, Johnson MA, Jordan KD (2005) Spectral signatures of hydrated proton vibrations in water clusters. Science 308:1765–69.
Communicated by Gnaiger Erich (2020-12-02) last update 2022-11-10
Isotopes of the hydrogen ion
- The most common isotope of hydrogen in nature contains a proton without any neutrons in the nucleus: 1H is called protium, distinguished from the second stable isotope deuterium 2H, and the third naturally occuring isotope is tritium 3H with a half-life of 12.32 years. The dissociation constant of heavy water (deuterium oxide) differs from normal water (Robinson et al 1969). 1H+ and 2H+ are hydrogen ions H+, but 2H+ is more than a proton. Respiratory complex IV and the other pumps in the electron transfer system pump not only protons, but pump 2H+.
Perspectives and questions for bioenergetic adventurers
- The following perspectives are considered in the 2020 edition of MitoPathways (Gnaiger 2020). There may be more to it:
- Protons (p+ or p; IUPAC symbols: Cohen et al 2008) in physics are nuclear subatomic particles. They are elementary particles, are visible, and are composed of invisible quarks. Their invisible counterparts are the electrons (IUPAC symbols e- or e).
- Protons occur besides variable numbers of neutrons in the nucleus of all atoms.
- Protons are stable in the hydrogen atoms of water molecules, and positively charged hydrogen ions contain one proton and zero or more neutrons depending on isotope type. Heavy water dissociates, but the neutron always goes together with the proton.
- Free hydrogen ions H+ are unstable in water. They are immediately hydrated to hydronium ions and higher degrees of hydration.
- It is convenient to summarize all these hydrated forms as H+. With respect to isotope differences, the generalized IUPAC term is 'hydron' (Bunnett, Jones 1988), which does not relate to ionic hydration species. The term 'hydrogenion' (with emphasis on the ion irrespective of hydration level) could be used for this meaning of H+, in contrast to hydrogen ion and proton (hydrion® and phydrion® are registered trademarks).
- The term proton is used in chemistry ambiguously for p and H+.
- This is more than confusing — is it even scary? Find out, when answering the following questions.
- See Hydrogen ion pump.
- Q1: Does the protonmotive force relate to protons or H+, or to both, or is there no difference?
- Q2: Do the proton pumps of the electron transfer system pump protons or hydrogen ions or hydronium ions?
- Q3: Do the proton pumps of the electron transfer system discriminate between hydrogen ions of different nuclear mass? Is there — as the name proton pump might suggest — any proton specificity at all?
- Q4: Do the proton pumps of the electron transfer system discriminate between protons electrically neutralized by electrons in a molecule, versus protons that are not electrically neutralized in a molecule?
- Q5: Do the proton pumps of the electron transfer system discriminate between protons in different molecules?
- Q6: What is the relative importance of protons and electrons in the mitochondrial electron transfer system?
- Q7: How can the meaning of the term protonmotive force be explained, when considering the above perspectives and questions?
References
- Bunnett JF, Jones RAY (1988) Names for hydrogen atoms, ions, and groups, and for reactions involving them. Pure Appl Chem 60:1115-16. - »Bioblast link«
- Cohen ER, Cvitas T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor HL (2008) Quantities, Units and Symbols in Physical Chemistry. IUPAC Green Book 3rd Edition, 2nd Printing, IUPAC & RSC Publishing, Cambridge. - »Bioblast link«
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. - »Bioblast link«
- Robinson RA, Paabo M, Bates RG (1969) Deuterium isotope effect on the dissociation of weak acids in water and deuterium oxide. J Res Natl Bur Stand A Phys Chem 73A:299-308. https://doi.org/10.6028/jres.073A.025. - »Bioblast link«
- Bioblast links: pH and protons - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- pH and protons
- » pH
- » hydrogen ion H+
- » hydron H+
- » hydronium ion H3O+
- » hydride H-
- » proton p+
- » pH buffering capacity
- » proton flux
- » proton pump versus hydrogen ion pump
- » proton leak
- » proton slip
- » protonmotive force
- pH and protons
- O2k-pH
- » O2k-Catalogue: O2k-pH ISE-Module
- » O2k-Manual pH electrode: MiPNet23.15 O2k-pH ISE-Module
- » O2k-SOP: MiPNet08.16 pH calibration
- » File:PH-Calibration-List.xls
- » NextGen-O2k, ratiometric: Carboxy SNARF 1
- » NextGen-O2k, ratiometric: HPTS
- » pH calibration buffers
- O2k-pH
- O2k-Publications
- HRFR - general
- » O2k-Manual: MiPNet22.11 O2k-FluoRespirometer manual
- » O2k signals and output
- » O2k-SOP: MiPNet14.06 Instrumental O2 background
- » MiPNet19.18A O2k-Series G: Start
- » ESD
- » O2k configuration
- » O2k control
- » O2k-FluoRespirometer
- » O2k-Main Unit#O2k-Series
- » Titration-Injection microPump
- » Compare: O2k-TPP+_ISE-Module
- HRFR - general
- DatLab
MitoPedia concepts:
Ergodynamics
MitoPedia topics:
Substrate and metabolite