Difference between revisions of "Cyclic voltammetry"
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=CV | |abbr=CV | ||
|description= '''Cyclic voltammetry''' (CV) is a type of electrochemical measurement which is applied | |description= '''Cyclic voltammetry''' (CV) is a type of electrochemical measurement which is applied with the [[Q-Module]] as quality control to | ||
(''1'') determine the oxidation and reduction peak potentials of [[Coenzyme Q]] in the specific experimental condition, (2) check the quality of the [[Q-Sensor]], and (''3'') test the interference of chemicals used in the HRR assay with the Q-Sensor. In CV, the [[Q-Sensor]] with the [[three-electrode system]] is used to obtain information about the analyte ([[Coenzyme Q|CoQ]]) by measuring the current (''I'') as the electric potential (''V'') between two of the electrodes is varied. In CV the electric potential between the glassy carbon (GC) and the Ag/AgCl reference electrode changes linearly versus time in cyclical phases, while the current is detected between GC and platinum electrode (Pt). The detected current is plotted versus the applied voltage to obtain the typical cyclic voltammogram trace (Figure 1). The presence of substances that are oxidized/reduced will result in current between GC and Pt, which can be seen as characteristic peaks in the voltammogram at a defined potential. The oxidation or the reduction peak potential values are used to set the GC (integrated into the [[Q-Sensor]]) for a separate experiment to measure the [[Q redox state]] of a biological sample. The oxidation and reduction peak potentials can be influenced by 1) the respiration medium, 2) the type of [[Coenzyme Q | CoQ]], 3) the polarization window, 4) the scan speed, 5) the number of cycles, 6) the concentration of the analyte (CoQ), and 7) the initial polarization voltage. <be> | |||
:::-''See'': [[MiPNet24.12 NextGen-O2k: Q-Module]]. | |||
}} | }} | ||
[[File:CV.png|center| | [[File:CV ox and red peak pot.png|center|900px]] | ||
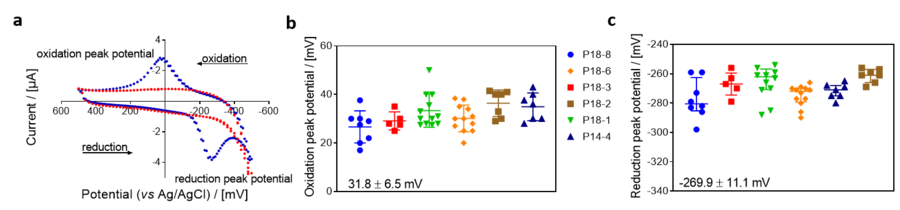
'''Figure 1.''' Cyclic voltammogram of [[ | '''Figure 1.''' Cyclic voltammogram in the absence and presence of [[coenzyme Q2]] (CoQ2) in non-stirred MiR05-Kit, at 37 °C by using the NextGen-O2k. Initial potential difference: +30 mV, polarization window: between -500 mV and +500 mV, scanning speed:100 mV/s, gain: 1. a: Blue plot: CV in the presence of CoQ2 (30 µM); red plot: background CV without CoQ2. Experiment: 2019-06-14_PQ2. Oxidation (b) and reduction (c) peak potentials of CoQ2 measured with cyclic voltammetry using different Q-Sensors. Codes of the Q-Sensors are next to the figure. Oxidation and reduction peak potentials are shown in the figure expressed as average ± SD [mV]; n=51. | ||
== Cyclic voltammetry is part of the [[Q-Module]] and the [[NextGen-O2k]] project == | == Cyclic voltammetry is part of the [[Q-Module]] and the [[NextGen-O2k]] project == | ||
Revision as of 18:20, 9 February 2021
Description
Cyclic voltammetry (CV) is a type of electrochemical measurement which is applied with the Q-Module as quality control to (1) determine the oxidation and reduction peak potentials of Coenzyme Q in the specific experimental condition, (2) check the quality of the Q-Sensor, and (3) test the interference of chemicals used in the HRR assay with the Q-Sensor. In CV, the Q-Sensor with the three-electrode system is used to obtain information about the analyte (CoQ) by measuring the current (I) as the electric potential (V) between two of the electrodes is varied. In CV the electric potential between the glassy carbon (GC) and the Ag/AgCl reference electrode changes linearly versus time in cyclical phases, while the current is detected between GC and platinum electrode (Pt). The detected current is plotted versus the applied voltage to obtain the typical cyclic voltammogram trace (Figure 1). The presence of substances that are oxidized/reduced will result in current between GC and Pt, which can be seen as characteristic peaks in the voltammogram at a defined potential. The oxidation or the reduction peak potential values are used to set the GC (integrated into the Q-Sensor) for a separate experiment to measure the Q redox state of a biological sample. The oxidation and reduction peak potentials can be influenced by 1) the respiration medium, 2) the type of CoQ, 3) the polarization window, 4) the scan speed, 5) the number of cycles, 6) the concentration of the analyte (CoQ), and 7) the initial polarization voltage. <be>
Abbreviation: CV
Figure 1. Cyclic voltammogram in the absence and presence of coenzyme Q2 (CoQ2) in non-stirred MiR05-Kit, at 37 °C by using the NextGen-O2k. Initial potential difference: +30 mV, polarization window: between -500 mV and +500 mV, scanning speed:100 mV/s, gain: 1. a: Blue plot: CV in the presence of CoQ2 (30 µM); red plot: background CV without CoQ2. Experiment: 2019-06-14_PQ2. Oxidation (b) and reduction (c) peak potentials of CoQ2 measured with cyclic voltammetry using different Q-Sensors. Codes of the Q-Sensors are next to the figure. Oxidation and reduction peak potentials are shown in the figure expressed as average ± SD [mV]; n=51.
Cyclic voltammetry is part of the Q-Module and the NextGen-O2k project
- The Q-Module allows for monitoring of the redox state of electron transfer-reactive coenzyme Q at the Q-junction using the specific Q-Stoppers with the integrated three-electrode system and the modified electronics of the NextGen-O2k. Cyclic voltammetry is used for quality control and for defining the polarization voltage applied during Q-redox measurements.
- Reference:
- Komlódi T, Cardoso LHD, Doerrier C, Moore AL, Rich PR, Gnaiger E (2021) Coupling and pathway control of coenzyme Q redox state and respiration in isolated mitochondria. Bioenerg Commun 2021.3. https://doi.org/10.26124/bec:2021-0003
- Reference:
Communicated by Komlodi T, Cardoso LHD 2020-07-28
- Bioblast links: Q - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Coenzyme Q
- » Coenzyme Q
- » Quinone, Ubiquinone Q; oxidized
- » Quinol, Ubiquinol QH2; reduced
- » Semiquinone
- » Coenzyme Q2
- » Q-redox state
- » Q-pools
- Coenzyme Q
- Mitochondrial pathways, respiratory Complexes, and Q
- » Q-cycle
- » Q-junction
- » Convergent electron flow
- » NS-pathway
- » FNS
- » FNSGp
- Mitochondrial pathways, respiratory Complexes, and Q
- NextGen-O2k and Q-Module