Gnaiger 2019 MitoFit Preprints: Difference between revisions
No edit summary |
No edit summary |
||
| (207 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{MitoFit page name}} | ||
{{Publication | {{Publication | ||
|title= | |title=Gnaiger E, Aasander Frostner E, Abdul Karim N, Abdel-Rahman EA, Abumrad NA, Acuna-Castroviejo D, Adiele RC, ''et al'' (2019) Mitochondrial respiratory states and rates. https://doi.org/10.26124/mitofit:190001.v6. — '''''Published''': 2020-05-20 Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1'' | ||
|info=MitoFit Preprint Arch 2019.1.v6 [[File:MitoFit Preprint Arch pdf.png|left|160px|link=https://www.mitofit.org/images/4/46/Gnaiger_2019_MitoFit_Preprint_Arch_doi_10.26124_mitofit_190001.pdf |MitoFit pdf]] '''[https://www.mitofit.org/images/4/46/Gnaiger_2019_MitoFit_Preprint_Arch_doi_10.26124_mitofit_190001.pdf Mitochondrial respiratory states and rates]''' | |||
|authors=''''' | <br /> | ||
| | <br /> | ||
|authors=MitoFit Prep 2019.1.v6. | |||
|year=2019 | |||
|journal=MitoFit Prep | |||
|abstract=Version 6 ('''v6''') '''2019-08-30''' [https://www.mitofit.org/images/4/46/Gnaiger_2019_MitoFit_Preprint_Arch_doi_10.26124_mitofit_190001.pdf doi:10.26124/mitofit:190001.v6] | |||
::: <small>Versions ('''v5''') 2019-07-24; ('''v4''') 2019-05-20; ('''v3''') 2019-04-24; ('''v2''') 2019-03-15; ('''v1''') 2019-02-12 - [https://www.mitofit.org/index.php/File:Gnaiger_2019_MitoFit_Preprint_Arch_doi_10.26124_mitofit_190001.pdf#Links_to_all_versions »Link to all versions«]</small> | |||
::: <big>'''Published online''': 2020-05-20 | |||
:::» Gnaiger Erich et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1. '''»[[BEC_2020.1 doi10.26124bec2020-0001.v1 |Bioblast link]]«'''</big> | |||
As the knowledge base and importance of mitochondrial physiology to human health expands, the necessity for harmonizing the terminology concerning mitochondrial respiratory states and rates has become increasingly apparent. The chemiosmotic theory establishes the mechanism of energy transformation and coupling in oxidative phosphorylation. The unifying concept of the protonmotive force provides the framework for developing a consistent theoretical foundation of mitochondrial physiology and bioenergetics. We follow guidelines of the International Union of Pure and Applied Chemistry (IUPAC) on terminology in physical chemistry, extended by considerations of open systems and thermodynamics of irreversible processes. The concept-driven constructive terminology incorporates the meaning of each quantity and aligns concepts and symbols to the nomenclature of classical bioenergetics. We endeavour to provide a balanced view on mitochondrial respiratory control and a critical discussion on reporting data of mitochondrial respiration in terms of metabolic flows and fluxes. Uniform standards for evaluation of respiratory states and rates will ultimately contribute to reproducibility between laboratories and thus support the development of databases of mitochondrial respiratory function in species, tissues, and cells. Clarity of concept and consistency of nomenclature facilitate effective transdisciplinary communication, education, and ultimately further discovery. | |||
|keywords=Mitochondrial respiratory control, coupling control, mitochondrial preparations, protonmotive force, uncoupling, oxidative phosphorylation, OXPHOS, efficiency, electron transfer, ET; proton leak, LEAK, residual oxygen consumption, ROX, State 2, State 3, State 4, normalization, flow, flux, O<sub>2</sub> | |keywords=Mitochondrial respiratory control, coupling control, mitochondrial preparations, protonmotive force, uncoupling, oxidative phosphorylation, OXPHOS, efficiency, electron transfer, ET; proton leak, LEAK, residual oxygen consumption, ROX, State 2, State 3, State 4, normalization, flow, flux, O<sub>2</sub> | ||
|editor=[[Gnaiger E]] | |editor=[[Gnaiger E]] | ||
}} | }} | ||
__TOC__ | __TOC__ | ||
== Authors: MitoEAGLE Task Group == | |||
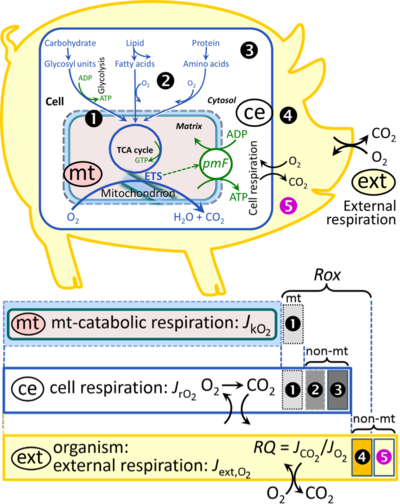
[[File:Respiration.png|thumb|right|400px|'''Figure 1. Internal and external respiration.''' Mitochondrial respiration is the oxidation of fuel substrates (electron donors) and reduction of O<sub>2</sub> catalysed by the electron transfer system, ETS: ('''mt''') mitochondrial catabolic respiration; ('''ce''') total cellular O<sub>2</sub> consumption; and ('''ext''') external respiration. All chemical reactions, r, that consume O<sub>2</sub> in the cells of an organism, contribute to cell respiration, ''J''<sub>rO2</sub>. In addition to mitochondrial catabolic respiration, O<sub>2</sub> is consumed by: (1) Mitochondrial residual oxygen consumption, ''Rox''. (2) Non-mitochondrial O<sub>2</sub> consumption by catabolic reactions, particularly peroxisomal oxidases and microsomal cytochrome P450 systems. (3) Non-mitochondrial ''Rox'' by reactions unrelated to catabolism. (4) Extracellular ''Rox''. (5) Aerobic microbial respiration. Bars are not at a quantitative scale.]] | [[File:Respiration.png|thumb|right|400px|'''Figure 1. Internal and external respiration.''' Mitochondrial respiration is the oxidation of fuel substrates (electron donors) and reduction of O<sub>2</sub> catalysed by the electron transfer system, ETS: ('''mt''') mitochondrial catabolic respiration; ('''ce''') total cellular O<sub>2</sub> consumption; and ('''ext''') external respiration. All chemical reactions, r, that consume O<sub>2</sub> in the cells of an organism, contribute to cell respiration, ''J''<sub>rO2</sub>. In addition to mitochondrial catabolic respiration, O<sub>2</sub> is consumed by: (1) Mitochondrial residual oxygen consumption, ''Rox''. (2) Non-mitochondrial O<sub>2</sub> consumption by catabolic reactions, particularly peroxisomal oxidases and microsomal cytochrome P450 systems. (3) Non-mitochondrial ''Rox'' by reactions unrelated to catabolism. (4) Extracellular ''Rox''. (5) Aerobic microbial respiration. Bars are not at a quantitative scale.]] | ||
:::* '''Corresponding author''' | |||
:::::: [[Gnaiger E |Erich Gnaiger]] | |||
:::::: Chair COST Action CA15203 MitoEAGLE | |||
:::::: T +43 512 566796 15, F +43 512 566796 20 | |||
:::::: [email protected] | www.mitoeagle.org | |||
:::* '''Coauthors''' (listed in alphabetical order); number of coauthors (2019-09-16): '''621''' | |||
:::::: ''Many coauthors have made significant additions and suggestions for improvement of the manuscript. All coauthors confirm to have read the final manuscript, and to agree to implement the recommendations into future manuscripts, presentations and teaching materials.'' | |||
:::::: Disclaimer: This article was prepared while Joshua P. Fessel was employed at Vanderbilt University Medical Center. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. | |||
:::* '''Copyright''': © 2019 Gnaiger et al. | |||
:::::: This is an [[Open Access]] [[preprint]] (not peer-reviewed) distributed under the terms of the [[Creative Commons Attribution License]], which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited. © remains with the authors, who have granted MitoFit an Open Access preprint licence in perpetuity. | |||
:::* '''Mailing list''' | |||
:::::: If you have read this paper and wish to be included in our mailing list, then see » [[MitoEAGLE#MitoEAGLE_Newsletter |MitoEAGLE Newsletter]] | |||
[[File:SI-units.png|left|120px]] | |||
== From Version 4 to 5 == | |||
On [https://www.euramet.org/publications-media-centre/news/?tx_news_pi1%5Bnews%5D=787&tx_news_pi1%5Baction%5D=detail&tx_news_pi1%5Bcontroller%5D=News World Metrology Day] (2019-05-20) the redefinition of the SI units came into force. At this occasion, Version 4 of the MitoEAGLE preprint on 'Mitochondrial respiratory rates and states' was released, in line with our emphasis on SI units for reporting data on mitochondrial respiratory physiology. | |||
[[ | == Preprints for [[Gentle Science]] == | ||
{{MitoFit preprint}} | |||
== References == | |||
(''' | :::# Altmann R (1894) Die Elementarorganismen und ihre Beziehungen zu den Zellen. Zweite vermehrte Auflage. Verlag Von Veit & Comp, Leipzig:160 pp. - [[Altmann 1894 Verlag Von Veit & Comp |»Bioblast link«]] | ||
:::# Baggeto LG, Testa-Perussini R (1990) Role of acetoin on the regulation of intermediate metabolism of Ehrlich ascites tumor mitochondria: its contribution to membrane cholesterol enrichment modifying passive proton permeability. Arch Biochem Biophys 283:341-8. | |||
:::# Beard DA (2005) A biophysical model of the mitochondrial respiratory system and oxidative phosphorylation. PLoS Comput Biol 1(4):e36. - [[Beard 2005 PLOS Comput Biol |»Bioblast link«]] | |||
:::# Benda C (1898) Weitere Mitteilungen über die Mitochondria. Verh Dtsch Physiol Ges:376-83. | |||
:::# Birkedal R, Laasmaa M, Vendelin M (2014) The location of energetic compartments affects energetic communication in cardiomyocytes. Front Physiol 5:376. | |||
:::# Blier PU, Dufresne F, Burton RS (2001) Natural selection and the evolution of mtDNA-encoded peptides: evidence for intergenomic co-adaptation. Trends Genet 17:400-6. | |||
:::# Blier PU, Guderley HE (1993) Mitochondrial activity in rainbow trout red muscle: the effect of temperature on the ADP-dependence of ATP synthesis. J Exp Biol 176:145-58. | |||
:::# Breton S, Beaupré HD, Stewart DT, Hoeh WR, Blier PU (2007) The unusual system of doubly uniparental inheritance of mtDNA: isn't one enough? Trends Genet 23:465-74. | |||
:::# Brown GC (1992) Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J 284:1-13. - [[Brown 1992 Biochem J |»Bioblast link«]] | |||
:::# Burger G, Gray MW, Forget L, Lang BF (2013) Strikingly bacteria-like and gene-rich mitochondrial genomes throughout jakobid protists. Genome Biol Evol 5:418-38. | |||
:::# Calvo SE, Klauser CR, Mootha VK (2016) MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Research 44:D1251-7. | |||
:::# Calvo SE, Julien O, Clauser KR, Shen H, Kamer KJ, Wells JA, Mootha VK (2017) Comparative analysis of mitochondrial N-termini from mouse, human, and yeast. Mol Cell Proteomics 16:512-23. | |||
:::# Campos JC, Queliconi BB, Bozi LHM, Bechara LRG, Dourado PMM, Andres AM, Jannig PR, Gomes KMS, Zambelli VO, Rocha-Resende C, Guatimosim S, Brum PC, Mochly-Rosen D, Gottlieb RA, Kowaltowski AJ, Ferreira JCB (2017) Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy 13:1304-317. - [[Campos 2017 Autophagy |»Bioblast link«]] | |||
:::# Canton M, Luvisetto S, Schmehl I, Azzone GF (1995) The nature of mitochondrial respiration and discrimination between membrane and pump properties. Biochem J 310:477-81. - [[Canton 1995 Biochem J |»Bioblast link«]] | |||
:::# Carrico C, Meyer JG, He W, Gibson BW, Verdin E (2018) The mitochondrial acylome emerges: proteomics, regulation by Sirtuins, and metabolic and disease implications. Cell Metab 27:497-512. - [[Carrico 2018 Cell Metab |»Bioblast link«]] | |||
:::# Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125:1241-52. - [[Chan 2006 Cell |»Bioblast link«]] | |||
:::# Chance B, Williams GR (1955a) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217:383-93. - [[Chance 1955 J Biol Chem-I |»Bioblast link«]] | |||
:::# Chance B, Williams GR (1955b) Respiratory enzymes in oxidative phosphorylation: III. The steady state. J Biol Chem 217:409-27. - [[Chance 1955 J Biol Chem-III |»Bioblast link«]] | |||
:::# Chance B, Williams GR (1955c) Respiratory enzymes in oxidative phosphorylation. IV. The respiratory chain. J Biol Chem 217:429-38. - [[Chance 1955 J Biol Chem-IV |»Bioblast link«]] | |||
:::# Chance B, Williams GR (1956) The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 17:65-134. - [[Chance 1956 Adv Enzymol Relat Subj Biochem |»Bioblast link«]] | |||
:::# Chowdhury SK, Djordjevic J, Albensi B, Fernyhough P (2015) Simultaneous evaluation of substrate-dependent oxygen consumption rates and mitochondrial membrane potential by TMRM and safranin in cortical mitochondria. Biosci Rep 36:e00286. - [[Chowdhury 2015 Biosci Rep |»Bioblast link«]] | |||
:::# Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, Mehta HH, Gao Q, Ashur C, Huffman DM, Wan J, Muzumdar R, Barzilai N, Cohen P (2016) Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY) 8:796-809. | |||
:::# Cohen ER, Cvitas T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor HL (2008) Quantities, units and smbols in physical chemistry, IUPAC Green Book, 3rd Edition, 2nd Printing, IUPAC & RSC Publishing, Cambridge. - [[Cohen 2008 IUPAC Green Book |»Bioblast link«]] | |||
:::# Cooper H, Hedges LV, Valentine JC, eds (2009) The handbook of research synthesis and meta-analysis. Russell Sage Foundation. | |||
:::# Coopersmith J (2010) Energy, the subtle concept. The discovery of Feynman’s blocks from Leibnitz to Einstein. Oxford University Press:400 pp. - [[Coopersmith 2010 Oxford Univ Press |»Bioblast link«]] | |||
:::# Cummins J (1998) Mitochondrial DNA in mammalian reproduction. Rev Reprod 3:172-82. | |||
:::# Dai Q, Shah AA, Garde RV, Yonish BA, Zhang L, Medvitz NA, Miller SE, Hansen EL, Dunn CN, Price TM (2013) A truncated progesterone receptor (PR-M) localizes to the mitochondrion and controls cellular respiration. Mol Endocrinol 27:741-53. | |||
:::# Daum B, Walter A, Horst A, Osiewacz HD, Kühlbrandt W (2013) Age-dependent dissociation of ATP synthase dimers and loss of inner-membrane cristae in mitochondria. Proc Natl Acad Sci U S A 110:15301-6. - [[Daum 2013 Proc Natl Acad Sci U S A |»Bioblast link«]] | |||
:::# Diebold LP, Gil HJ, Gao P, Martinez CA, Weinberg SE, Chandel NS (2019) Mitochondrial Complex III is necessary for endothelial cell proliferation during angiogenesis. Nat Metab 1:158–71. - [[Diebold 2019 Nat Metab |»Bioblast link«]] | |||
:::# Divakaruni AS, Brand MD (2011) The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda) 26:192-205. | |||
:::# Doerrier C, Garcia-Souza LF, Krumschnabel G, Wohlfarter Y, Mészáros AT, Gnaiger E (2018) High-Resolution FluoRespirometry and OXPHOS protocols for human cells, permeabilized fibres from small biopsies of muscle, and isolated mitochondria. Methods Mol Biol 1782 (Palmeira CM, Moreno AJ, eds): Mitochondrial Bioenergetics, 978-1-4939-7830-4. - [[Doerrier 2018 Methods Mol Biol |»Bioblast link«]] | |||
:::# Doskey CM, van ‘t Erve TJ, Wagner BA, Buettner GR (2015) Moles of a substance per cell is a highly informative dosing metric in cell culture. PLoS One 10:e0132572. | |||
:::# Drahota Z, Milerová M, Stieglerová A, Houstek J, Ostádal B (2004) Developmental changes of cytochrome c oxidase and citrate synthase in rat heart homogenate. Physiol Res 53:119-22. | |||
:::# Duarte FV, Palmeira CM, Rolo AP (2014) The role of microRNAs in mitochondria: small players acting wide. Genes (Basel) 5:865-86. | |||
:::# Ehinger JK, Morota S, Hansson MJ, Paul G, Elmér E (2015) Mitochondrial dysfunction in blood cells from amyotrophic lateral sclerosis patients. J Neurol 262:1493-503. - [[Ehinger 2015 J Neurol |»Bioblast link«]] | |||
:::# Ehinger JK, Piel S, Ford R, Karlsson M, Sjövall F, Frostner EÅ, Morota S, Taylor RW, Turnbull DM, Cornell C, Moss SJ, Metzsch C, Hansson MJ, Fliri H, Elmér E (2016) Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat Commun 7:12317. - [[Ehinger 2016 Nat Commun |»Bioblast link«]] | |||
:::# Ernster L, Schatz G (1981) Mitochondria: a historical review. J Cell Biol 91:227s-55s. - [[Ernster 1981 J Cell Biol |»Bioblast link«]] | |||
:::# Estabrook RW (1967) Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. Methods Enzymol 10:41-7. - [[Estabrook 1967 Methods Enzymol |»Bioblast link«]] | |||
:::# Faber C, Zhu ZJ, Castellino S, Wagner DS, Brown RH, Peterson RA, Gates L, Barton J, Bickett M, Hagerty L, Kimbrough C, Sola M, Bailey D, Jordan H, Elangbam CS (2014) Cardiolipin profiles as a potential biomarker of mitochondrial health in diet-induced obese mice subjected to exercise, diet-restriction and ephedrine treatment. J Appl Toxicol 34:1122-9. | |||
:::# Feagin JE, Harrell MI, Lee JC, Coe KJ, Sands BH, Cannone JJ, Tami G, Schnare MN, Gutell RR (2012) The fragmented mitochondrial ribosomal RNAs of ''Plasmodium falciparum''. PLoS One 7:e38320. | |||
:::# Fell D (1997) Understanding the control of metabolism. Portland Press. - [https://www.researchgate.net/publication/317369476_Understanding_the_Control_of_Metabolism »ResearchGate«] | |||
:::# Forstner H, Gnaiger E (1983) Calculation of equilibrium oxygen concentration. In: Polarographic Oxygen Sensors. Aquatic and Physiological Applications. Gnaiger E, Forstner H (eds), Springer, Berlin, Heidelberg, New York:321-33. - [[Forstner 1983 POS |»Bioblast link«]] | |||
:::# Garlid KD, Beavis AD, Ratkje SK (1989) On the nature of ion leaks in energy-transducing membranes. Biochim Biophys Acta 976:109-20. - [[Garlid 1989 Biochim Biophys Acta |»Bioblast link«]] | |||
:::# Garlid KD, Semrad C, Zinchenko V. Does redox slip contribute significantly to mitochondrial respiration? In: Schuster S, Rigoulet M, Ouhabi R, Mazat J-P, eds (1993) Modern trends in biothermokinetics. Plenum Press, New York, London:287-93. - [[Garlid 1993 BTK |»Bioblast link«]] | |||
:::# Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, Rehling P, Meisinger C, Ryan MT, Wiedemann N, Greenberg ML, Pfanner N (2009) Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol 19:2133-9. - [[Gebert 2009 Curr Biol |»Bioblast link«]] | |||
:::# Gerö D, Szabo C (2016) Glucocorticoids suppress mitochondrial oxidant production via upregulation of uncoupling protein 2 in hyperglycemic endothelial cells. PLoS One 11:e0154813. | |||
:::# Gibney E (2018) Largest overhaul of scientific units since 1875 wins approval. Nature 563:451–2. - [https://www.nature.com/articles/d41586-018-07424-8 nature.com] | |||
:::# Gnaiger E (1993a) Efficiency and power strategies under hypoxia. Is low efficiency at high glycolytic ATP production a paradox? In: Surviving Hypoxia: Mechanisms of Control and Adaptation. Hochachka PW, Lutz PL, Sick T, Rosenthal M, Van den Thillart G, eds. CRC Press, Boca Raton, Ann Arbor, London, Tokyo:77-109. - [[Gnaiger 1993 Hypoxia |»Bioblast link«]] | |||
:::# Gnaiger E (1993b) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65:1983-2002. - [[Gnaiger 1993 Pure Appl Chem |»Bioblast link«]] | |||
:::# Gnaiger E (2001) Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir Physiol 128:277-97. - [[Gnaiger 2001 Respir Physiol |»Bioblast link«]] | |||
:::# Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. - [[Gnaiger 2009 Int J Biochem Cell Biol |»Bioblast link«]] | |||
:::# Gnaiger E (2014) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 4th ed. Mitochondr Physiol Network 19.12. Oroboros MiPNet Publications, Innsbruck:80 pp. - [[Gnaiger 2014 MitoPathways |»Bioblast link«]] | |||
:::# Gnaiger E, Méndez G, Hand SC (2000) High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci USA 97:11080-5. - [[Gnaiger 2000 Proc Natl Acad Sci U S A |»Bioblast link«]] | |||
:::# Greggio C, Jha P, Kulkarni SS, Lagarrigue S, Broskey NT, Boutant M, Wang X, Conde Alonso S, Ofori E, Auwerx J, Cantó C, Amati F (2017) Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab 25:301-11. - [[Greggio 2017 Cell Metab |»Bioblast link«]] | |||
:::# Hinkle PC (2005) P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 1706:1-11. - [[Hinkle 2005 Biochim Biophys Acta |»Bioblast link«]] | |||
:::# Hofstadter DR (1979) Gödel, Escher, Bach: An eternal golden braid. A metaphorical fugue on minds and machines in the spirit of Lewis Carroll. Harvester Press:499 pp. - [[Hofstadter 1979 Harvester Press |»Bioblast link«]] | |||
:::# Illaste A, Laasmaa M, Peterson P, Vendelin M (2012) Analysis of molecular movement reveals latticelike obstructions to diffusion in heart muscle cells. Biophys J 102:739-48. | |||
:::# Jasienski M, Bazzaz FA (1999) The fallacy of ratios and the testability of models in biology. Oikos 84:321-26. | |||
:::# Jepihhina N, Beraud N, Sepp M, Birkedal R, Vendelin M (2011) Permeabilized rat cardiomyocyte response demonstrates intracellular origin of diffusion obstacles. Biophys J 101:2112-21. | |||
:::# Jezek P, Holendova B, Garlid KD, Jaburek M (2018) Mitochondrial uncoupling proteins: subtle regulators of cellular redox signaling. Antioxid Redox Signal 29:667-714. - [[Jezek 2018 Antioxid Redox Signal |»Bioblast link«]] | |||
:::# Karnkowska A, Vacek V, Zubáčová Z, Treitli SC, Petrželková R, Eme L, Novák L, Žárský V, Barlow LD, Herman EK, Soukal P, Hroudová M, Doležal P, Stairs CW, Roger AJ, Eliáš M, Dacks JB, Vlček Č, Hampl V (2016) A eukaryote without a mitochondrial organelle. Curr Biol 26:1274-84. | |||
:::# Kenwood BM, Weaver JL, Bajwa A, Poon IK, Byrne FL, Murrow BA, Calderone JA, Huang L, Divakaruni AS, Tomsig JL, Okabe K, Lo RH, Cameron Coleman G, Columbus L, Yan Z, Saucerman JJ, Smith JS, Holmes JW, Lynch KR, Ravichandran KS, Uchiyama S, Santos WL, Rogers GW, Okusa MD, Bayliss DA, Hoehn KL (2013) Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol Metab 3:114-23. - [[Kenwood 2013 Mol Metab |»Bioblast link«]] | |||
:::# Klepinin A, Ounpuu L, Guzun R, Chekulayev V, Timohhina N, Tepp K, Shevchuk I, Schlattner U, Kaambre T (2016) Simple oxygraphic analysis for the presence of adenylate kinase 1 and 2 in normal and tumor cells. J Bioenerg Biomembr 48:531-48. - [[Klepinin 2016 J Bioenerg Biomembr |»Bioblast link«]] | |||
:::# Koit A, Shevchuk I, Ounpuu L, Klepinin A, Chekulayev V, Timohhina N, Tepp K, Puurand M,Truu L, Heck K, Valvere V, Guzun R, Kaambre T (2017) Mitochondrial respiration in human colorectal and breast cancer clinical material is regulated differently. Oxid Med Cell Longev 1372640. - [[Koit 2017 Oxid Med Cell Longev |»Bioblast link«]] | |||
:::# Komlódi T, Tretter L (2017) Methylene blue stimulates substrate-level phosphorylation catalysed by succinyl-CoA ligase in the citric acid cycle. Neuropharmacology 123:287-98. - [[Komlodi 2017 Neuropharmacology |»Bioblast link«]] | |||
:::# Korn E (1969) Cell membranes: structure and synthesis. Annu Rev Biochem 38:263–88. | |||
:::# Lai N, M Kummitha C, Rosca MG, Fujioka H, Tandler B, Hoppel CL (2018) Isolation of mitochondrial subpopulations from skeletal muscle: optimizing recovery and preserving integrity. Acta Physiol (Oxf):e13182. doi: 10.1111/apha.13182. | |||
:::# Lane N (2005) Power, sex, suicide: mitochondria and the meaning of life. Oxford University Press:354 pp. - [[Lane 2005 Oxford Univ Press |»Bioblast link«]] | |||
:::# Larsen S, Nielsen J, Neigaard Nielsen C, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel RC, Helge JW, Dela F, Hey-Mogensen M (2012) Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590:3349-60. - [[Larsen 2012 J Physiol |»Bioblast link«]] | |||
:::# Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P (2015) The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21:443-54. | |||
:::# Lee SR, Kim HK, Song IS, Youm J, Dizon LA, Jeong SH, Ko TH, Heo HJ, Ko KS, Rhee BD, Kim N, Han J (2013) Glucocorticoids and their receptors: insights into specific roles in mitochondria. Prog Biophys Mol Biol 112:44-54. | |||
:::# Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS (2001) Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 280:R441-7. | |||
:::# Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B (1998) The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366:177-96. - [[Lemasters 1998 Biochim Biophys Acta |»Bioblast link«]] | |||
:::# Lemieux H, Blier PU, Gnaiger E (2017) Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep 7:2840. - [[Lemieux 2017 Sci Rep |»Bioblast link«]] | |||
:::# Lemieux H, Semsroth S, Antretter H, Höfer D, Gnaiger E (2011) Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol 43:1729–38. - [[Lemieux 2011 Int J Biochem Cell Biol |»Bioblast link«]] | |||
:::# Lenaz G, Tioli G, Falasca AI, Genova ML (2017) Respiratory supercomplexes in mitochondria. In: Mechanisms of primary energy trasduction in biology. M Wikstrom (ed) Royal Society of Chemistry Publishing, London, UK:296-337. | |||
:::# Ling C, Rönn T (2019) Epigenetics in human obesity and type 2 diabetes. Cell Metab 29:1028-44. https://doi.org/10.1016/j.cmet.2019.03.009. - [[Ling 2019 Cell Metab |»Bioblast link«]] | |||
:::# Lisowski P, Kannan P, Mlody B, Prigione A (2018) Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep 19:e45432. - [[Lisowski 2018 EMBO Rep |»Bioblast link«]] | |||
:::# Liu S, Roellig DM, Guo Y, Li N, Frace MA, Tang K, Zhang L, Feng Y, Xiao L (2016) Evolution of mitosome metabolism and invasion-related proteins in Cryptosporidium. BMC Genomics 17:1006. | |||
:::# Luo S, Valencia CA, Zhang J, Lee NC, Slone J, Gui B, Wang X, Li Z, Dell S, Brown J, Chen SM, Chien YH, Hwu WL, Fan PC, Wong LJ, Atwal PS, Huang T (2018) Biparental inheritance of mitochondrial DNA in humans. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1810946115. - [[Luo 2018 Proc Natl Acad Sci U S A |»Bioblast link«]] | |||
:::# Margulis L (1970) Origin of eukaryotic cells. New Haven: Yale University Press. | |||
:::# McDonald AE, Vanlerberghe GC, Staples JF (2009) Alternative oxidase in animals: unique characteristics and taxonomic distribution. J Exp Biol 212:2627-34. | |||
:::# McKenzie M, Lazarou M, Thorburn DR, Ryan MT (2006) Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol 361:462-9. - [[McKenzie 2006 J Mol Biol |»Bioblast link«]] | |||
:::# Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH (2006) Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61:534-40. | |||
:::# Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE (2007) Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol (1985) 103:21-7. | |||
:::# Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE (2005) Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab 288:E818-25. | |||
:::# Miller GA (1991) The science of words. Scientific American Library New York:276 pp. - [[Miller 1991 Scientific American Library |»Bioblast link«]] | |||
:::# Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144-8. - [[Mitchell 1961 Nature |»Bioblast link«]] | |||
:::# Mitchell P (2011) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta Bioenergetics 1807:1507-38. - [[Mitchell 2011 Biochim Biophys Acta |»Bioblast link«]] | |||
:::# Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K (2007) Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56:1592-9. | |||
:::# Mohr PJ, Phillips WD (2015) Dimensionless units in the SI. Metrologia 52:40-7. | |||
:::# Moreno M, Giacco A, Di Munno C, Goglia F (2017) Direct and rapid effects of 3,5-diiodo-L-thyronine (T2). Mol Cell Endocrinol 7207:30092-8. | |||
:::# Morrow RM, Picard M, Derbeneva O, Leipzig J, McManus MJ, Gouspillou G, Barbat-Artigas S, Dos Santos C, Hepple RT, Murdock DG, Wallace DC (2017) Mitochondrial energy deficiency leads to hyperproliferation of skeletal muscle mitochondria and enhanced insulin sensitivity. Proc Natl Acad Sci U S A 114:2705-10. - [[Morrow 2017 Proc Natl Acad Sci U S A |»Bioblast link«]] | |||
:::# Murley A, Nunnari J (2016) The emerging network of mitochondria-organelle contacts. Mol Cell 61:648-53. | |||
:::# National Academies of Sciences, Engineering, and Medicine (2018) International coordination for science data infrastructure: Proceedings of a workshop—in brief. Washington, DC: The National Academies Press. doi: https://doi.org/10.17226/25015. - [[National Academies of Sciences, Engineering, and Medicine 2018 Science data infrastructure |»Bioblast link«]] | |||
:::# Oemer G, Lackner L, Muigg K, Krumschnabel G, Watschinger K, Sailer S, Lindner H, Gnaiger E, Wortmann SB, Werner ER, Zschocke J, Keller MA (2018) The molecular structural diversity of mitochondrial cardiolipins. Proc Nat Acad Sci U S A 115:4158-63. - [[Oemer 2018 Proc Nat Acad Sci U S A |»Bioblast link«]] | |||
:::# Palmfeldt J, Bross P (2017) Proteomics of human mitochondria. Mitochondrion 33:2-14. | |||
:::# Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G (2014) Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837:408-17. - [[Paradies 2014 Biochim Biophys Acta |»Bioblast link«]] | |||
:::# Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibres from small biopsies of human muscle. Methods Mol Biol 810:25-58. - [[Pesta 2012 Methods Mol Biol |»Bioblast link«]] | |||
:::# Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E (2011) Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301:R1078–87. - [[Pesta 2011 Am J Physiol Regul Integr Comp Physiol |»Bioblast link«]] | |||
:::# Price TM, Dai Q (2015) The role of a mitochondrial progesterone receptor (PR-M) in progesterone action. Semin Reprod Med 33:185-94. | |||
:::# Puchowicz MA, Varnes ME, Cohen BH, Friedman NR, Kerr DS, Hoppel CL (2004) Oxidative phosphorylation analysis: assessing the integrated functional activity of human skeletal muscle mitochondria – case studies. Mitochondrion 4:377-85. - [[Puchowicz 2004 Mitochondrion |»Bioblast link«]] | |||
:::# Puntschart A, Claassen H, Jostarndt K, Hoppeler H, Billeter R (1995) mRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am J Physiol 269:C619-25. | |||
:::# Quiros PM, Mottis A, Auwerx J (2016) Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol 17:213-26. | |||
:::# Rackham O, Mercer TR, Filipovska A (2012) The human mitochondrial transcriptome and the RNA-binding proteins that regulate its expression. WIREs RNA 3:675–95. | |||
:::# Rackham O, Shearwood AM, Mercer TR, Davies SM, Mattick JS, Filipovska A (2011) Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 17:2085-93. - [[Rackham 2011 RNA |»Bioblast link«]] | |||
:::# Reichmann H, Hoppeler H, Mathieu-Costello O, von Bergen F, Pette D (1985) Biochemical and ultrastructural changes of skeletal muscle mitochondria after chronic electrical stimulation in rabbits. Pflugers Arch 404:1-9. | |||
:::# Renner K, Amberger A, Konwalinka G, Gnaiger E (2003) Changes of mitochondrial respiration, mitochondrial content and cell size after induction of apoptosis in leukemia cells. Biochim Biophys Acta 1642:115-23. - [[Renner 2003 Biochim Biophys Acta |»Bioblast link«]] | |||
:::# Rice DW, Alverson AJ, Richardson AO, Young GJ, Sanchez-Puerta MV, Munzinger J, Barry K, Boore JL, Zhang Y, dePamphilis CW, Knox EB, Palmer JD (2016) Horizontal transfer of entire genomes via mitochondrial fusion in the angiosperm Amborella. Science 342:1468-73. | |||
:::# Rich P (2003) Chemiosmotic coupling: The cost of living. Nature 421:583. - [[Rich 2003 Nature |»Bioblast link«]] | |||
:::# Rich PR (2013) Chemiosmotic theory. Encyclopedia Biol Chem 1:467-72. - [[Rich 2013 Encyclopedia Biol Chem |»Bioblast link«]] | |||
:::# Roger JA, Munoz-Gomes SA, Kamikawa R (2017) The origin and diversification of mitochondria. Curr Biol 27:R1177-92. | |||
:::# Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL (2008) Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci USA 105:18746-51. - [[Rostovtseva 2008 Proc Natl Acad Sci U S A |»Bioblast link«]] | |||
:::# Rustin P, Parfait B, Chretien D, Bourgeron T, Djouadi F, Bastin J, Rötig A, Munnich A (1996) Fluxes of nicotinamide adenine dinucleotides through mitochondrial membranes in human cultured cells. J Biol Chem 271:14785-90. | |||
:::# Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS (1998) Permeabilised cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 184:81-100. - [[Saks 1998 Mol Cell Biochem |»Bioblast link«]] | |||
:::# Salabei JK, Gibb AA, Hill BG (2014) Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat Protoc 9:421-38. | |||
:::# Sazanov LA (2015) A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat Rev Mol Cell Biol 16:375-88. - [[Sazanov 2015 Nat Rev Mol Cell Biol |»Bioblast link«]] | |||
:::# Schneider TD (2006) Claude Shannon: biologist. The founder of information theory used biology to formulate the channel capacity. IEEE Eng Med Biol Mag 25:30-3. | |||
:::# Schönfeld P, Dymkowska D, Wojtczak L (2009) Acyl-CoA-induced generation of reactive oxygen species in mitochondrial preparations is due to the presence of peroxisomes. Free Radic Biol Med 47:503-9. - [[Schoenfeld 2009 Free Radic Biol Med |»Bioblast link«]] | |||
:::# Schultz J, Wiesner RJ (2000) Proliferation of mitochondria in chronically stimulated rabbit skeletal muscle--transcription of mitochondrial genes and copy number of mitochondrial DNA. J Bioenerg Biomembr 32:627-34. | |||
:::# Simson P, Jepihhina N, Laasmaa M, Peterson P, Birkedal R, Vendelin M (2016) Restricted ADP movement in cardiomyocytes: Cytosolic diffusion obstacles are complemented with a small number of open mitochondrial voltage-dependent anion channels. J Mol Cell Cardiol 97:197-203. | |||
:::# Singh BK, Sinha RA, Tripathi M, Mendoza A, Ohba K, Sy JAC, Xie SY, Zhou J, Ho JP, Chang CY, Wu Y, Giguère V, Bay BH, Vanacker JM, Ghosh S, Gauthier K, Hollenberg AN, McDonnell DP, Yen PM (2018) Thyroid hormone receptor and ERRα coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci Signal 11(536) DOI: 10.1126/scisignal.aam5855. - [[Singh 2018 Sci Signal |»Bioblast link«]] | |||
:::# Speijer D (2016) Being right on Q: shaping eukaryotic evolution. Biochem J 473:4103-27. - [[Speijer 2016 Biochem J |»Bioblast link«]] | |||
:::# Stucki JW, Ineichen EA (1974) Energy dissipation by calcium recycling and the efficiency of calcium transport in rat-liver mitochondria. Eur J Biochem 48:365-75. | |||
:::# Sugiura A, Mattie S, Prudent J, McBride HM (2017) Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 542:251-4. - [[Sugiura 2017 Nature |»Bioblast link«]] | |||
:::# Tonkonogi M, Harris B, Sahlin K (1997) Increased activity of citrate synthase in human skeletal muscle after a single bout of prolonged exercise. Acta Physiol Scand 161:435-6. | |||
:::# Torralba D, Baixauli F, Sánchez-Madrid F (2016) Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front Cell Dev Biol 4:107. eCollection 2016. - [[Torralba 2016 Front Cell Dev Biol |»Bioblast link«]] | |||
:::# Vamecq J, Schepers L, Parmentier G, Mannaerts GP (1987) Inhibition of peroxisomal fatty acyl-CoA oxidase by antimycin A. Biochem J 248:603-7. - [[Vamecq 1987 Biochem J |»Bioblast link«]] | |||
:::# Waczulikova I, Habodaszova D, Cagalinec M, Ferko M, Ulicna O, Mateasik A, Sikurova L, Ziegelhöffer A (2007) Mitochondrial membrane fluidity, potential, and calcium transients in the myocardium from acute diabetic rats. Can J Physiol Pharmacol 85:372-81. | |||
:::# Wagner BA, Venkataraman S, Buettner GR (2011) The rate of oxygen utilization by cells. Free Radic Biol Med 51:700-712. | |||
:::# Wang H, Hiatt WR, Barstow TJ, Brass EP (1999) Relationships between muscle mitochondrial DNA content, mitochondrial enzyme activity and oxidative capacity in man: alterations with disease. Eur J Appl Physiol Occup Physiol 80:22-7. | |||
:::# Watt IN, Montgomery MG, Runswick MJ, Leslie AG, Walker JE (2010) Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci U S A 107:16823-7. - [[Watt 2010 Proc Natl Acad Sci U S A |»Bioblast link«]] | |||
:::# Weibel ER, Hoppeler H (2005) Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J Exp Biol 208:1635–44. | |||
:::# White DJ, Wolff JN, Pierson M, Gemmell NJ (2008) Revealing the hidden complexities of mtDNA inheritance. Mol Ecol 17:4925–42. | |||
:::# Wikström M, Hummer G (2012) Stoichiometry of proton translocation by respiratory complex I and its mechanistic implications. Proc Natl Acad Sci U S A 109:4431-6. - [[Wikstroem 2012 Proc Natl Acad Sci U S A |»Bioblast link«]] | |||
:::# Williams EG, Wu Y, Jha P, Dubuis S, Blattmann P, Argmann CA, Houten SM, Amariuta T, Wolski W, Zamboni N, Aebersold R, Auwerx J (2016) Systems proteomics of liver mitochondria function. Science 352 (6291):aad0189 | |||
:::# Willis WT, Jackman MR, Messer JI, Kuzmiak-Glancy S, Glancy B (2016) A simple hydraulic analog model of oxidative phosphorylation. Med Sci Sports Exerc 48:990-1000. | |||
:::# Yoshinaga MY, Kellermann MY, Valentine DL, Valentine RC (2016) Phospholipids and glycolipids mediate proton containment and circulation along the surface of energy-transducing membranes. Prog Lipid Res 64:1-15. | |||
:::# Zíková A, Hampl V, Paris Z, Týč J, Lukeš J (2016) Aerobic mitochondria of parasitic protists: diverse genomes and complex functions. Mol Biochem Parasitol 209:46-57. | |||
== Corrigendum == | |||
::::* Section 2.5.4: Sulfur dioxygenase is related to ET, hence should not be listed here in relation to ''Rox''. | |||
== | == Comments and communication == | ||
::::» [[Talk:Gnaiger_2019_MitoFit_Preprint_Arch#2019-07-22_Circular_to_coauthors |2019-07-22 Circular to coauthors]] | |||
::::» [[Talk:Gnaiger_2019_MitoFit_Preprint_Arch#2019-03-12_Circular_to_coauthors |2019-03-12 Circular to coauthors]] | |||
::::» [[Talk:Gnaiger_2019_MitoFit_Preprint_Arch#2019-02-12_Circular_to_coauthors |2019-02-12 Circular to coauthors]] | |||
::::* [[Talk:Gnaiger_2019_MitoFit_Preprint_Arch#Comments |Comments]] | |||
== Linking COST Actions and MiP''society'' == | |||
== | :::: [[File:MITOEAGLE-logo.jpg|60px|link=http://www.bioblast.at/index.php/COST_Action_MitoEAGLE|COST Action MitoEAGLE]] [[File:COST Logo.jpg|160px|link=http://www.cost.eu/COST_Actions/ca/CA15203|e-COST MitoEAGLE]] [[File:EU-logo.jpg|80px|link=http://www.cost.eu/COST_Actions/ca/CA15203?parties|e-COST MitoEAGLE countries]] [[Image:MiPsocietyLOGO.JPG|100px|link=http://www.mitoglobal.org/index.php/Mitochondrial_Physiology_Society|MiP''society'']] | ||
:::: | |||
:: | |||
: | |||
= | |||
: | |||
::::* [http://www.cost.eu/COST_Actions/ca/CA15203 COST Action CA15203 MitoEAGLE] | ::::* [http://www.cost.eu/COST_Actions/ca/CA15203 COST Action CA15203 MitoEAGLE] | ||
::::* [http://www.cost.eu/COST_Actions/ca/CA16225 COST Action CA16225 EU-CARDIOPROTECTION] | ::::* [http://www.cost.eu/COST_Actions/ca/CA16225 COST Action CA16225 EU-CARDIOPROTECTION] | ||
| Line 166: | Line 207: | ||
== The MitoFit preprint == | |||
{{MitoEAGLE preprint 1 Phases}} | {{MitoEAGLE preprint 1 Phases}} | ||
{{NextGen-O2k H2020-support}} | |||
::::* [http://www.mitoeagle.org/index.php/File:MitoEAGLE_preprint_States_and_rates.pdf Versions towards the MitoFit Preprint] | |||
::::* [http://www.mitoeagle.org/index.php/Talk:MitoEAGLE_Task_Group_States_and_rates Discussion towards the MitoFit Preprint] | |||
== Cited by == | |||
{{Template:Cited by Gnaiger 2019 MitoFit Preprints}} | |||
== | |||
: | |||
}} | |||
Latest revision as of 08:27, 8 January 2023
Gnaiger 2019 MitoFit Preprints
| Gnaiger E, Aasander Frostner E, Abdul Karim N, Abdel-Rahman EA, Abumrad NA, Acuna-Castroviejo D, Adiele RC, et al (2019) Mitochondrial respiratory states and rates. https://doi.org/10.26124/mitofit:190001.v6. — Published: 2020-05-20 Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1 |
» MitoFit Preprint Arch 2019.1.v6
Mitochondrial respiratory states and rates
MitoFit Prep 2019.1.v6. (2019) MitoFit Prep
Abstract: Version 6 (v6) 2019-08-30 doi:10.26124/mitofit:190001.v6
- Versions (v5) 2019-07-24; (v4) 2019-05-20; (v3) 2019-04-24; (v2) 2019-03-15; (v1) 2019-02-12 - »Link to all versions«
- Published online: 2020-05-20
- » Gnaiger Erich et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1. »Bioblast link«
As the knowledge base and importance of mitochondrial physiology to human health expands, the necessity for harmonizing the terminology concerning mitochondrial respiratory states and rates has become increasingly apparent. The chemiosmotic theory establishes the mechanism of energy transformation and coupling in oxidative phosphorylation. The unifying concept of the protonmotive force provides the framework for developing a consistent theoretical foundation of mitochondrial physiology and bioenergetics. We follow guidelines of the International Union of Pure and Applied Chemistry (IUPAC) on terminology in physical chemistry, extended by considerations of open systems and thermodynamics of irreversible processes. The concept-driven constructive terminology incorporates the meaning of each quantity and aligns concepts and symbols to the nomenclature of classical bioenergetics. We endeavour to provide a balanced view on mitochondrial respiratory control and a critical discussion on reporting data of mitochondrial respiration in terms of metabolic flows and fluxes. Uniform standards for evaluation of respiratory states and rates will ultimately contribute to reproducibility between laboratories and thus support the development of databases of mitochondrial respiratory function in species, tissues, and cells. Clarity of concept and consistency of nomenclature facilitate effective transdisciplinary communication, education, and ultimately further discovery. • Keywords: Mitochondrial respiratory control, coupling control, mitochondrial preparations, protonmotive force, uncoupling, oxidative phosphorylation, OXPHOS, efficiency, electron transfer, ET; proton leak, LEAK, residual oxygen consumption, ROX, State 2, State 3, State 4, normalization, flow, flux, O2 • Bioblast editor: Gnaiger E
Authors: MitoEAGLE Task Group
- Corresponding author
- Erich Gnaiger
- Chair COST Action CA15203 MitoEAGLE
- T +43 512 566796 15, F +43 512 566796 20
- [email protected] | www.mitoeagle.org
- Coauthors (listed in alphabetical order); number of coauthors (2019-09-16): 621
- Many coauthors have made significant additions and suggestions for improvement of the manuscript. All coauthors confirm to have read the final manuscript, and to agree to implement the recommendations into future manuscripts, presentations and teaching materials.
- Disclaimer: This article was prepared while Joshua P. Fessel was employed at Vanderbilt University Medical Center. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
- Copyright: © 2019 Gnaiger et al.
- This is an Open Access preprint (not peer-reviewed) distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited. © remains with the authors, who have granted MitoFit an Open Access preprint licence in perpetuity.
- Mailing list
- If you have read this paper and wish to be included in our mailing list, then see » MitoEAGLE Newsletter
From Version 4 to 5
On World Metrology Day (2019-05-20) the redefinition of the SI units came into force. At this occasion, Version 4 of the MitoEAGLE preprint on 'Mitochondrial respiratory rates and states' was released, in line with our emphasis on SI units for reporting data on mitochondrial respiratory physiology.
Preprints for Gentle Science
» MitoFit Preprints - the Open Access preprint server for mitochondrial physiology and bioenergetics
References
- Altmann R (1894) Die Elementarorganismen und ihre Beziehungen zu den Zellen. Zweite vermehrte Auflage. Verlag Von Veit & Comp, Leipzig:160 pp. - »Bioblast link«
- Baggeto LG, Testa-Perussini R (1990) Role of acetoin on the regulation of intermediate metabolism of Ehrlich ascites tumor mitochondria: its contribution to membrane cholesterol enrichment modifying passive proton permeability. Arch Biochem Biophys 283:341-8.
- Beard DA (2005) A biophysical model of the mitochondrial respiratory system and oxidative phosphorylation. PLoS Comput Biol 1(4):e36. - »Bioblast link«
- Benda C (1898) Weitere Mitteilungen über die Mitochondria. Verh Dtsch Physiol Ges:376-83.
- Birkedal R, Laasmaa M, Vendelin M (2014) The location of energetic compartments affects energetic communication in cardiomyocytes. Front Physiol 5:376.
- Blier PU, Dufresne F, Burton RS (2001) Natural selection and the evolution of mtDNA-encoded peptides: evidence for intergenomic co-adaptation. Trends Genet 17:400-6.
- Blier PU, Guderley HE (1993) Mitochondrial activity in rainbow trout red muscle: the effect of temperature on the ADP-dependence of ATP synthesis. J Exp Biol 176:145-58.
- Breton S, Beaupré HD, Stewart DT, Hoeh WR, Blier PU (2007) The unusual system of doubly uniparental inheritance of mtDNA: isn't one enough? Trends Genet 23:465-74.
- Brown GC (1992) Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J 284:1-13. - »Bioblast link«
- Burger G, Gray MW, Forget L, Lang BF (2013) Strikingly bacteria-like and gene-rich mitochondrial genomes throughout jakobid protists. Genome Biol Evol 5:418-38.
- Calvo SE, Klauser CR, Mootha VK (2016) MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Research 44:D1251-7.
- Calvo SE, Julien O, Clauser KR, Shen H, Kamer KJ, Wells JA, Mootha VK (2017) Comparative analysis of mitochondrial N-termini from mouse, human, and yeast. Mol Cell Proteomics 16:512-23.
- Campos JC, Queliconi BB, Bozi LHM, Bechara LRG, Dourado PMM, Andres AM, Jannig PR, Gomes KMS, Zambelli VO, Rocha-Resende C, Guatimosim S, Brum PC, Mochly-Rosen D, Gottlieb RA, Kowaltowski AJ, Ferreira JCB (2017) Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy 13:1304-317. - »Bioblast link«
- Canton M, Luvisetto S, Schmehl I, Azzone GF (1995) The nature of mitochondrial respiration and discrimination between membrane and pump properties. Biochem J 310:477-81. - »Bioblast link«
- Carrico C, Meyer JG, He W, Gibson BW, Verdin E (2018) The mitochondrial acylome emerges: proteomics, regulation by Sirtuins, and metabolic and disease implications. Cell Metab 27:497-512. - »Bioblast link«
- Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125:1241-52. - »Bioblast link«
- Chance B, Williams GR (1955a) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217:383-93. - »Bioblast link«
- Chance B, Williams GR (1955b) Respiratory enzymes in oxidative phosphorylation: III. The steady state. J Biol Chem 217:409-27. - »Bioblast link«
- Chance B, Williams GR (1955c) Respiratory enzymes in oxidative phosphorylation. IV. The respiratory chain. J Biol Chem 217:429-38. - »Bioblast link«
- Chance B, Williams GR (1956) The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 17:65-134. - »Bioblast link«
- Chowdhury SK, Djordjevic J, Albensi B, Fernyhough P (2015) Simultaneous evaluation of substrate-dependent oxygen consumption rates and mitochondrial membrane potential by TMRM and safranin in cortical mitochondria. Biosci Rep 36:e00286. - »Bioblast link«
- Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, Mehta HH, Gao Q, Ashur C, Huffman DM, Wan J, Muzumdar R, Barzilai N, Cohen P (2016) Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY) 8:796-809.
- Cohen ER, Cvitas T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor HL (2008) Quantities, units and smbols in physical chemistry, IUPAC Green Book, 3rd Edition, 2nd Printing, IUPAC & RSC Publishing, Cambridge. - »Bioblast link«
- Cooper H, Hedges LV, Valentine JC, eds (2009) The handbook of research synthesis and meta-analysis. Russell Sage Foundation.
- Coopersmith J (2010) Energy, the subtle concept. The discovery of Feynman’s blocks from Leibnitz to Einstein. Oxford University Press:400 pp. - »Bioblast link«
- Cummins J (1998) Mitochondrial DNA in mammalian reproduction. Rev Reprod 3:172-82.
- Dai Q, Shah AA, Garde RV, Yonish BA, Zhang L, Medvitz NA, Miller SE, Hansen EL, Dunn CN, Price TM (2013) A truncated progesterone receptor (PR-M) localizes to the mitochondrion and controls cellular respiration. Mol Endocrinol 27:741-53.
- Daum B, Walter A, Horst A, Osiewacz HD, Kühlbrandt W (2013) Age-dependent dissociation of ATP synthase dimers and loss of inner-membrane cristae in mitochondria. Proc Natl Acad Sci U S A 110:15301-6. - »Bioblast link«
- Diebold LP, Gil HJ, Gao P, Martinez CA, Weinberg SE, Chandel NS (2019) Mitochondrial Complex III is necessary for endothelial cell proliferation during angiogenesis. Nat Metab 1:158–71. - »Bioblast link«
- Divakaruni AS, Brand MD (2011) The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda) 26:192-205.
- Doerrier C, Garcia-Souza LF, Krumschnabel G, Wohlfarter Y, Mészáros AT, Gnaiger E (2018) High-Resolution FluoRespirometry and OXPHOS protocols for human cells, permeabilized fibres from small biopsies of muscle, and isolated mitochondria. Methods Mol Biol 1782 (Palmeira CM, Moreno AJ, eds): Mitochondrial Bioenergetics, 978-1-4939-7830-4. - »Bioblast link«
- Doskey CM, van ‘t Erve TJ, Wagner BA, Buettner GR (2015) Moles of a substance per cell is a highly informative dosing metric in cell culture. PLoS One 10:e0132572.
- Drahota Z, Milerová M, Stieglerová A, Houstek J, Ostádal B (2004) Developmental changes of cytochrome c oxidase and citrate synthase in rat heart homogenate. Physiol Res 53:119-22.
- Duarte FV, Palmeira CM, Rolo AP (2014) The role of microRNAs in mitochondria: small players acting wide. Genes (Basel) 5:865-86.
- Ehinger JK, Morota S, Hansson MJ, Paul G, Elmér E (2015) Mitochondrial dysfunction in blood cells from amyotrophic lateral sclerosis patients. J Neurol 262:1493-503. - »Bioblast link«
- Ehinger JK, Piel S, Ford R, Karlsson M, Sjövall F, Frostner EÅ, Morota S, Taylor RW, Turnbull DM, Cornell C, Moss SJ, Metzsch C, Hansson MJ, Fliri H, Elmér E (2016) Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat Commun 7:12317. - »Bioblast link«
- Ernster L, Schatz G (1981) Mitochondria: a historical review. J Cell Biol 91:227s-55s. - »Bioblast link«
- Estabrook RW (1967) Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. Methods Enzymol 10:41-7. - »Bioblast link«
- Faber C, Zhu ZJ, Castellino S, Wagner DS, Brown RH, Peterson RA, Gates L, Barton J, Bickett M, Hagerty L, Kimbrough C, Sola M, Bailey D, Jordan H, Elangbam CS (2014) Cardiolipin profiles as a potential biomarker of mitochondrial health in diet-induced obese mice subjected to exercise, diet-restriction and ephedrine treatment. J Appl Toxicol 34:1122-9.
- Feagin JE, Harrell MI, Lee JC, Coe KJ, Sands BH, Cannone JJ, Tami G, Schnare MN, Gutell RR (2012) The fragmented mitochondrial ribosomal RNAs of Plasmodium falciparum. PLoS One 7:e38320.
- Fell D (1997) Understanding the control of metabolism. Portland Press. - »ResearchGate«
- Forstner H, Gnaiger E (1983) Calculation of equilibrium oxygen concentration. In: Polarographic Oxygen Sensors. Aquatic and Physiological Applications. Gnaiger E, Forstner H (eds), Springer, Berlin, Heidelberg, New York:321-33. - »Bioblast link«
- Garlid KD, Beavis AD, Ratkje SK (1989) On the nature of ion leaks in energy-transducing membranes. Biochim Biophys Acta 976:109-20. - »Bioblast link«
- Garlid KD, Semrad C, Zinchenko V. Does redox slip contribute significantly to mitochondrial respiration? In: Schuster S, Rigoulet M, Ouhabi R, Mazat J-P, eds (1993) Modern trends in biothermokinetics. Plenum Press, New York, London:287-93. - »Bioblast link«
- Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, Rehling P, Meisinger C, Ryan MT, Wiedemann N, Greenberg ML, Pfanner N (2009) Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol 19:2133-9. - »Bioblast link«
- Gerö D, Szabo C (2016) Glucocorticoids suppress mitochondrial oxidant production via upregulation of uncoupling protein 2 in hyperglycemic endothelial cells. PLoS One 11:e0154813.
- Gibney E (2018) Largest overhaul of scientific units since 1875 wins approval. Nature 563:451–2. - nature.com
- Gnaiger E (1993a) Efficiency and power strategies under hypoxia. Is low efficiency at high glycolytic ATP production a paradox? In: Surviving Hypoxia: Mechanisms of Control and Adaptation. Hochachka PW, Lutz PL, Sick T, Rosenthal M, Van den Thillart G, eds. CRC Press, Boca Raton, Ann Arbor, London, Tokyo:77-109. - »Bioblast link«
- Gnaiger E (1993b) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65:1983-2002. - »Bioblast link«
- Gnaiger E (2001) Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir Physiol 128:277-97. - »Bioblast link«
- Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. - »Bioblast link«
- Gnaiger E (2014) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 4th ed. Mitochondr Physiol Network 19.12. Oroboros MiPNet Publications, Innsbruck:80 pp. - »Bioblast link«
- Gnaiger E, Méndez G, Hand SC (2000) High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci USA 97:11080-5. - »Bioblast link«
- Greggio C, Jha P, Kulkarni SS, Lagarrigue S, Broskey NT, Boutant M, Wang X, Conde Alonso S, Ofori E, Auwerx J, Cantó C, Amati F (2017) Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab 25:301-11. - »Bioblast link«
- Hinkle PC (2005) P/O ratios of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 1706:1-11. - »Bioblast link«
- Hofstadter DR (1979) Gödel, Escher, Bach: An eternal golden braid. A metaphorical fugue on minds and machines in the spirit of Lewis Carroll. Harvester Press:499 pp. - »Bioblast link«
- Illaste A, Laasmaa M, Peterson P, Vendelin M (2012) Analysis of molecular movement reveals latticelike obstructions to diffusion in heart muscle cells. Biophys J 102:739-48.
- Jasienski M, Bazzaz FA (1999) The fallacy of ratios and the testability of models in biology. Oikos 84:321-26.
- Jepihhina N, Beraud N, Sepp M, Birkedal R, Vendelin M (2011) Permeabilized rat cardiomyocyte response demonstrates intracellular origin of diffusion obstacles. Biophys J 101:2112-21.
- Jezek P, Holendova B, Garlid KD, Jaburek M (2018) Mitochondrial uncoupling proteins: subtle regulators of cellular redox signaling. Antioxid Redox Signal 29:667-714. - »Bioblast link«
- Karnkowska A, Vacek V, Zubáčová Z, Treitli SC, Petrželková R, Eme L, Novák L, Žárský V, Barlow LD, Herman EK, Soukal P, Hroudová M, Doležal P, Stairs CW, Roger AJ, Eliáš M, Dacks JB, Vlček Č, Hampl V (2016) A eukaryote without a mitochondrial organelle. Curr Biol 26:1274-84.
- Kenwood BM, Weaver JL, Bajwa A, Poon IK, Byrne FL, Murrow BA, Calderone JA, Huang L, Divakaruni AS, Tomsig JL, Okabe K, Lo RH, Cameron Coleman G, Columbus L, Yan Z, Saucerman JJ, Smith JS, Holmes JW, Lynch KR, Ravichandran KS, Uchiyama S, Santos WL, Rogers GW, Okusa MD, Bayliss DA, Hoehn KL (2013) Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol Metab 3:114-23. - »Bioblast link«
- Klepinin A, Ounpuu L, Guzun R, Chekulayev V, Timohhina N, Tepp K, Shevchuk I, Schlattner U, Kaambre T (2016) Simple oxygraphic analysis for the presence of adenylate kinase 1 and 2 in normal and tumor cells. J Bioenerg Biomembr 48:531-48. - »Bioblast link«
- Koit A, Shevchuk I, Ounpuu L, Klepinin A, Chekulayev V, Timohhina N, Tepp K, Puurand M,Truu L, Heck K, Valvere V, Guzun R, Kaambre T (2017) Mitochondrial respiration in human colorectal and breast cancer clinical material is regulated differently. Oxid Med Cell Longev 1372640. - »Bioblast link«
- Komlódi T, Tretter L (2017) Methylene blue stimulates substrate-level phosphorylation catalysed by succinyl-CoA ligase in the citric acid cycle. Neuropharmacology 123:287-98. - »Bioblast link«
- Korn E (1969) Cell membranes: structure and synthesis. Annu Rev Biochem 38:263–88.
- Lai N, M Kummitha C, Rosca MG, Fujioka H, Tandler B, Hoppel CL (2018) Isolation of mitochondrial subpopulations from skeletal muscle: optimizing recovery and preserving integrity. Acta Physiol (Oxf):e13182. doi: 10.1111/apha.13182.
- Lane N (2005) Power, sex, suicide: mitochondria and the meaning of life. Oxford University Press:354 pp. - »Bioblast link«
- Larsen S, Nielsen J, Neigaard Nielsen C, Nielsen LB, Wibrand F, Stride N, Schroder HD, Boushel RC, Helge JW, Dela F, Hey-Mogensen M (2012) Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 590:3349-60. - »Bioblast link«
- Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P (2015) The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21:443-54.
- Lee SR, Kim HK, Song IS, Youm J, Dizon LA, Jeong SH, Ko TH, Heo HJ, Ko KS, Rhee BD, Kim N, Han J (2013) Glucocorticoids and their receptors: insights into specific roles in mitochondria. Prog Biophys Mol Biol 112:44-54.
- Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS (2001) Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 280:R441-7.
- Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B (1998) The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366:177-96. - »Bioblast link«
- Lemieux H, Blier PU, Gnaiger E (2017) Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep 7:2840. - »Bioblast link«
- Lemieux H, Semsroth S, Antretter H, Höfer D, Gnaiger E (2011) Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol 43:1729–38. - »Bioblast link«
- Lenaz G, Tioli G, Falasca AI, Genova ML (2017) Respiratory supercomplexes in mitochondria. In: Mechanisms of primary energy trasduction in biology. M Wikstrom (ed) Royal Society of Chemistry Publishing, London, UK:296-337.
- Ling C, Rönn T (2019) Epigenetics in human obesity and type 2 diabetes. Cell Metab 29:1028-44. https://doi.org/10.1016/j.cmet.2019.03.009. - »Bioblast link«
- Lisowski P, Kannan P, Mlody B, Prigione A (2018) Mitochondria and the dynamic control of stem cell homeostasis. EMBO Rep 19:e45432. - »Bioblast link«
- Liu S, Roellig DM, Guo Y, Li N, Frace MA, Tang K, Zhang L, Feng Y, Xiao L (2016) Evolution of mitosome metabolism and invasion-related proteins in Cryptosporidium. BMC Genomics 17:1006.
- Luo S, Valencia CA, Zhang J, Lee NC, Slone J, Gui B, Wang X, Li Z, Dell S, Brown J, Chen SM, Chien YH, Hwu WL, Fan PC, Wong LJ, Atwal PS, Huang T (2018) Biparental inheritance of mitochondrial DNA in humans. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1810946115. - »Bioblast link«
- Margulis L (1970) Origin of eukaryotic cells. New Haven: Yale University Press.
- McDonald AE, Vanlerberghe GC, Staples JF (2009) Alternative oxidase in animals: unique characteristics and taxonomic distribution. J Exp Biol 212:2627-34.
- McKenzie M, Lazarou M, Thorburn DR, Ryan MT (2006) Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol 361:462-9. - »Bioblast link«
- Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH (2006) Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 61:534-40.
- Menshikova EV, Ritov VB, Ferrell RE, Azuma K, Goodpaster BH, Kelley DE (2007) Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J Appl Physiol (1985) 103:21-7.
- Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE (2005) Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab 288:E818-25.
- Miller GA (1991) The science of words. Scientific American Library New York:276 pp. - »Bioblast link«
- Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144-8. - »Bioblast link«
- Mitchell P (2011) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta Bioenergetics 1807:1507-38. - »Bioblast link«
- Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Højlund K (2007) Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56:1592-9.
- Mohr PJ, Phillips WD (2015) Dimensionless units in the SI. Metrologia 52:40-7.
- Moreno M, Giacco A, Di Munno C, Goglia F (2017) Direct and rapid effects of 3,5-diiodo-L-thyronine (T2). Mol Cell Endocrinol 7207:30092-8.
- Morrow RM, Picard M, Derbeneva O, Leipzig J, McManus MJ, Gouspillou G, Barbat-Artigas S, Dos Santos C, Hepple RT, Murdock DG, Wallace DC (2017) Mitochondrial energy deficiency leads to hyperproliferation of skeletal muscle mitochondria and enhanced insulin sensitivity. Proc Natl Acad Sci U S A 114:2705-10. - »Bioblast link«
- Murley A, Nunnari J (2016) The emerging network of mitochondria-organelle contacts. Mol Cell 61:648-53.
- National Academies of Sciences, Engineering, and Medicine (2018) International coordination for science data infrastructure: Proceedings of a workshop—in brief. Washington, DC: The National Academies Press. doi: https://doi.org/10.17226/25015. - »Bioblast link«
- Oemer G, Lackner L, Muigg K, Krumschnabel G, Watschinger K, Sailer S, Lindner H, Gnaiger E, Wortmann SB, Werner ER, Zschocke J, Keller MA (2018) The molecular structural diversity of mitochondrial cardiolipins. Proc Nat Acad Sci U S A 115:4158-63. - »Bioblast link«
- Palmfeldt J, Bross P (2017) Proteomics of human mitochondria. Mitochondrion 33:2-14.
- Paradies G, Paradies V, De Benedictis V, Ruggiero FM, Petrosillo G (2014) Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837:408-17. - »Bioblast link«
- Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibres from small biopsies of human muscle. Methods Mol Biol 810:25-58. - »Bioblast link«
- Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, Parson W, Burtscher M, Schocke M, Gnaiger E (2011) Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol 301:R1078–87. - »Bioblast link«
- Price TM, Dai Q (2015) The role of a mitochondrial progesterone receptor (PR-M) in progesterone action. Semin Reprod Med 33:185-94.
- Puchowicz MA, Varnes ME, Cohen BH, Friedman NR, Kerr DS, Hoppel CL (2004) Oxidative phosphorylation analysis: assessing the integrated functional activity of human skeletal muscle mitochondria – case studies. Mitochondrion 4:377-85. - »Bioblast link«
- Puntschart A, Claassen H, Jostarndt K, Hoppeler H, Billeter R (1995) mRNAs of enzymes involved in energy metabolism and mtDNA are increased in endurance-trained athletes. Am J Physiol 269:C619-25.
- Quiros PM, Mottis A, Auwerx J (2016) Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol 17:213-26.
- Rackham O, Mercer TR, Filipovska A (2012) The human mitochondrial transcriptome and the RNA-binding proteins that regulate its expression. WIREs RNA 3:675–95.
- Rackham O, Shearwood AM, Mercer TR, Davies SM, Mattick JS, Filipovska A (2011) Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 17:2085-93. - »Bioblast link«
- Reichmann H, Hoppeler H, Mathieu-Costello O, von Bergen F, Pette D (1985) Biochemical and ultrastructural changes of skeletal muscle mitochondria after chronic electrical stimulation in rabbits. Pflugers Arch 404:1-9.
- Renner K, Amberger A, Konwalinka G, Gnaiger E (2003) Changes of mitochondrial respiration, mitochondrial content and cell size after induction of apoptosis in leukemia cells. Biochim Biophys Acta 1642:115-23. - »Bioblast link«
- Rice DW, Alverson AJ, Richardson AO, Young GJ, Sanchez-Puerta MV, Munzinger J, Barry K, Boore JL, Zhang Y, dePamphilis CW, Knox EB, Palmer JD (2016) Horizontal transfer of entire genomes via mitochondrial fusion in the angiosperm Amborella. Science 342:1468-73.
- Rich P (2003) Chemiosmotic coupling: The cost of living. Nature 421:583. - »Bioblast link«
- Rich PR (2013) Chemiosmotic theory. Encyclopedia Biol Chem 1:467-72. - »Bioblast link«
- Roger JA, Munoz-Gomes SA, Kamikawa R (2017) The origin and diversification of mitochondria. Curr Biol 27:R1177-92.
- Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL (2008) Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci USA 105:18746-51. - »Bioblast link«
- Rustin P, Parfait B, Chretien D, Bourgeron T, Djouadi F, Bastin J, Rötig A, Munnich A (1996) Fluxes of nicotinamide adenine dinucleotides through mitochondrial membranes in human cultured cells. J Biol Chem 271:14785-90.
- Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS (1998) Permeabilised cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 184:81-100. - »Bioblast link«
- Salabei JK, Gibb AA, Hill BG (2014) Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat Protoc 9:421-38.
- Sazanov LA (2015) A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat Rev Mol Cell Biol 16:375-88. - »Bioblast link«
- Schneider TD (2006) Claude Shannon: biologist. The founder of information theory used biology to formulate the channel capacity. IEEE Eng Med Biol Mag 25:30-3.
- Schönfeld P, Dymkowska D, Wojtczak L (2009) Acyl-CoA-induced generation of reactive oxygen species in mitochondrial preparations is due to the presence of peroxisomes. Free Radic Biol Med 47:503-9. - »Bioblast link«
- Schultz J, Wiesner RJ (2000) Proliferation of mitochondria in chronically stimulated rabbit skeletal muscle--transcription of mitochondrial genes and copy number of mitochondrial DNA. J Bioenerg Biomembr 32:627-34.
- Simson P, Jepihhina N, Laasmaa M, Peterson P, Birkedal R, Vendelin M (2016) Restricted ADP movement in cardiomyocytes: Cytosolic diffusion obstacles are complemented with a small number of open mitochondrial voltage-dependent anion channels. J Mol Cell Cardiol 97:197-203.
- Singh BK, Sinha RA, Tripathi M, Mendoza A, Ohba K, Sy JAC, Xie SY, Zhou J, Ho JP, Chang CY, Wu Y, Giguère V, Bay BH, Vanacker JM, Ghosh S, Gauthier K, Hollenberg AN, McDonnell DP, Yen PM (2018) Thyroid hormone receptor and ERRα coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci Signal 11(536) DOI: 10.1126/scisignal.aam5855. - »Bioblast link«
- Speijer D (2016) Being right on Q: shaping eukaryotic evolution. Biochem J 473:4103-27. - »Bioblast link«
- Stucki JW, Ineichen EA (1974) Energy dissipation by calcium recycling and the efficiency of calcium transport in rat-liver mitochondria. Eur J Biochem 48:365-75.
- Sugiura A, Mattie S, Prudent J, McBride HM (2017) Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 542:251-4. - »Bioblast link«
- Tonkonogi M, Harris B, Sahlin K (1997) Increased activity of citrate synthase in human skeletal muscle after a single bout of prolonged exercise. Acta Physiol Scand 161:435-6.
- Torralba D, Baixauli F, Sánchez-Madrid F (2016) Mitochondria know no boundaries: mechanisms and functions of intercellular mitochondrial transfer. Front Cell Dev Biol 4:107. eCollection 2016. - »Bioblast link«
- Vamecq J, Schepers L, Parmentier G, Mannaerts GP (1987) Inhibition of peroxisomal fatty acyl-CoA oxidase by antimycin A. Biochem J 248:603-7. - »Bioblast link«
- Waczulikova I, Habodaszova D, Cagalinec M, Ferko M, Ulicna O, Mateasik A, Sikurova L, Ziegelhöffer A (2007) Mitochondrial membrane fluidity, potential, and calcium transients in the myocardium from acute diabetic rats. Can J Physiol Pharmacol 85:372-81.
- Wagner BA, Venkataraman S, Buettner GR (2011) The rate of oxygen utilization by cells. Free Radic Biol Med 51:700-712.
- Wang H, Hiatt WR, Barstow TJ, Brass EP (1999) Relationships between muscle mitochondrial DNA content, mitochondrial enzyme activity and oxidative capacity in man: alterations with disease. Eur J Appl Physiol Occup Physiol 80:22-7.
- Watt IN, Montgomery MG, Runswick MJ, Leslie AG, Walker JE (2010) Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria. Proc Natl Acad Sci U S A 107:16823-7. - »Bioblast link«
- Weibel ER, Hoppeler H (2005) Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J Exp Biol 208:1635–44.
- White DJ, Wolff JN, Pierson M, Gemmell NJ (2008) Revealing the hidden complexities of mtDNA inheritance. Mol Ecol 17:4925–42.
- Wikström M, Hummer G (2012) Stoichiometry of proton translocation by respiratory complex I and its mechanistic implications. Proc Natl Acad Sci U S A 109:4431-6. - »Bioblast link«
- Williams EG, Wu Y, Jha P, Dubuis S, Blattmann P, Argmann CA, Houten SM, Amariuta T, Wolski W, Zamboni N, Aebersold R, Auwerx J (2016) Systems proteomics of liver mitochondria function. Science 352 (6291):aad0189
- Willis WT, Jackman MR, Messer JI, Kuzmiak-Glancy S, Glancy B (2016) A simple hydraulic analog model of oxidative phosphorylation. Med Sci Sports Exerc 48:990-1000.
- Yoshinaga MY, Kellermann MY, Valentine DL, Valentine RC (2016) Phospholipids and glycolipids mediate proton containment and circulation along the surface of energy-transducing membranes. Prog Lipid Res 64:1-15.
- Zíková A, Hampl V, Paris Z, Týč J, Lukeš J (2016) Aerobic mitochondria of parasitic protists: diverse genomes and complex functions. Mol Biochem Parasitol 209:46-57.
Corrigendum
- Section 2.5.4: Sulfur dioxygenase is related to ET, hence should not be listed here in relation to Rox.
Comments and communication
Linking COST Actions and MiPsociety
The MitoFit preprint
- » Manuscript phases and versions
Manuscript phases and versions - an open-access apporach
- This manuscript on ‘Mitochondrial respiratory states and rates’ is a position statement in the frame of COST Action CA15203 MitoEAGLE. The list of coauthors evolved beyond phase 1 in the bottom-up spirit of COST.
- The global MitoEAGLE network made it possible to collaborate with a large number of coauthors to reach consensus on the present manuscript. Nevertheless, we do not consider scientific progress to be supported by ‘declaration’ statements (other than on ethical or political issues). Our manuscript aims at providing arguments for further debate rather than pushing opinions. We hope to initiate a much broader process of discussion and want to raise the awareness on the importance of a consistent terminology for reporting of scientific data in the field of bioenergetics, mitochondrial physiology and pathology. Quality of research requires quality of communication. Some established researchers in the field may not want to re-consider the use of jargon which has become established despite deficiencies of accuracy and meaning. In the long run, superior standards will become accepted. We hope to contribute to this evolutionary process, with an emphasis on harmonization rather than standardization.
- Phase 1: The protonmotive force and respiratory control
- » The protonmotive force and respiratory control - Discussion
- 2016-11 MitoEAGLE Verona 2016
- 2017-03 MitoEAGLE Barcelona 2017
- 2017-04-09 to 2017-09-18 (44 versions)
- 2017-07 MiPschool Obergurgl 2017
- 2017-09-21 to 2018-02-06 (21 versions)
- » MitoEAGLE preprint 2017-09-21 - Discussion
- 2017-11-11: Print version (16) for MiP2017/MitoEAGLE Hradec Kralove CZ
- Phase 2: Mitochondrial respiratory states and rates: Building blocks of mitochondrial physiology Part 1
- » MitoEAGLE Task Group States and rates - Discussion
- 2018-02-08 (42 versions up to present)
- 2018-08 EBEC2018 Budapest HU - Poster
- 2018-09 MiP2018/MitoEAGLE Jurmala LV
- 2018-10 MiPschool Tromso-Bergen 2018
- Phase 3: 2019-02-12: Publication as a preprint: MitoFit Preprints with DOI number, providing widely accepted visible proof that the publication is citable. - See MitoFit DOI Data Center (2018-12-12 submission).
- Phase 4: Journal submission
- Target: CELL METABOLISM, aiming at indexing by The Web of Science and PubMed.
- Coauthors
- 2017-09-21 Version 01: 105 coauthors
- 2017-10-15 Version 10: 131 coauthors
- 2018-01-18 Version 20: 168 coauthors
- 2018-02-26 Version 30: 225 coauthors
- 2018-08-20 Version 40: 350 coauthors - EBEC Poster
- 2018-10-17 Version 44: 426 coauthors - MiPschool Tromso-Bergen 2018
- 2018-12-12 Version 50: 517 coauthors - Submission to the preprint server bioRxiv not successful
- 2019-02-12 Preprint version 1: 530 coauthors
- 2019-03-15 Preprint version 2: 533 coauthors
- 2019-04-24 Preprint version 3: 533 coauthors
- 2019-05-20 Preprint version 4: 542 coauthors
- 2019-07-24 Preprint version 5: 612 coauthors
- 2019-08-30 Preprint version 6: 622 coauthors - Preprint publication doi:10.26124/mitofit:190001.v6
- Coauthors
- BEC 2020.1. - Gnaiger Erich et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1. - »Bioblast link«
Cited by
- Gnaiger E (2019) Editorial: A vision on preprints for mitochondrial physiology and bioenergetics. MitoFit Preprint Arch doi:10.26124/mitofit:190002.v2.









