| News and Events | Working Groups | Short-Term Scientific Missions | Management Committee | Members |
COST Action CA15203 (2016-2021): MitoEAGLE
Evolution-Age-Gender-Lifestyle-Environment: mitochondrial fitness mapping
Gnaiger 2019 MitoFit Preprints
| MitoEAGLE preprint 2018-03-03(33) Mitochondrial respiratory states and rates: Building blocks of mitochondrial physiology
Part 1. - http://www.mitoeagle.org/index.php/MitoEAGLE_preprint_2018-02-08. |
» Last update 2018-03-03:  - » Versions
- » Versions
Corresponding author:, Gnaiger E, Co-authors:, Aasander Frostner E, Acuna-Castroviejo D, Ahn B, Alves MG, Amati F, Aral C, Arandarcikaite O, Bailey DM, Bakker BM, Bastos Sant'Anna Silva AC, Battino M, Beard DA, Ben-Shachar D, Bishop D, Borsheim E, Borutaite V, Bouillaud F, Breton S, Brown GC, Brown RA, Buettner GR, Burtscher J, Calabria E, Calbet JA, Calzia E, Cardoso LHD, Carvalho E, Casado Pinna M, Cervinkova Z, Chang SC, Chaurasia B, Chen Q, Chicco AJ, Chinopoulos C, Clementi E, Coen PM, Collin A, Crisostomo L, Das AM, Davis MS, De Palma C, Dias TR, Distefano G, Doerrier C, Drahota Z, Duchen MR, Durham WJ, Ehinger J, Elmer E, Endlicher R, Fell DA, Ferko M, Ferreira JCB, Ferreira R, Filipovska A, Fisar Z, Fischer M, Fisher JJ, Fornaro M, Galkin A, Garcia-Roves PM, Garcia-Souza LF, Garten A, Genova ML, Giovarelli M, Gonzalez-Armenta JL, Gonzalo H, Goodpaster BH, Gorr TA, Gourlay CW, Granata C, Grefte S, Haas CB, Haavik J, Han J, Harrison DK, Hellgren KT, Hernansanz-Agustin P, Holland OJ, Hoppel CL, Houstek J, Hunger M, Iglesias-Gonzalez J, Irving BA, Iyer S, Jackson CB, Jadiya P, Jang DH, Jansen-Duerr P, Jespersen NR, Jha RK, Jurk D, Kaambre T, Kane DA, Kappler L, Karabatsiakis A, Keijer J, Keppner G, Khamoui AV, Klingenspor M, Komlodi T, Koopman WJH, Kopitar-Jerala N, Krajcova A, Krako Jakovljevic N, Kuang J, Kucera O, Labieniec-Watala M, Lai N, Laner V, Larsen TS, Lee HK, Lemieux H, Leeuwenburgh C, Lerfall J, Liu J, Lucchinetti E, Macedo MP, MacMillan-Crow LA, Makrecka-Kuka M, Malik AN, Markova M, Menze MA, Meszaros AT, Michalak S, Moisoi N, Molina AJA, Montaigne D, Moore AL, Mracek T, Muntane J, Muntean DM, Murray AJ, Nemec M, Neuzil J, Newsom S, Nozickova K, O'Gorman D, Oliveira PF, Oliveira MT, Oliveira PJ, Orynbayeva Z, Pak YK, Palmeira CM, Passos JF, Patel HH, Pecina P, Pelnena D, Pereira da Silva Grilo da Silva F, Pesta D, Petit PX, Pichaud N, Piel S, Pirkmajer S, Porter RK, Pranger F, Prochownik EV, Pulinilkunnil T, Puurand M, Radenkovic F, Radi R, Ramzan R, Reboredo P, Renner-Sattler K, Robinson MM, Roesland GV, Rohlena J, Rolo AP, Ropelle ER, Rossiter HB, Rybacka-Mossakowska J, Saada A, Safaei Z, Salin K, Salvadego D, Sandi C, Scatena R, Schartner M, Scheibye-Knudsen M, Schilling JM, Schlattner U, Schoenfeld P, Schwarzer C, Scott GR, Shabalina IG, Sharma P, Sharma V, Shevchuk I, Siewiera K, Silber AM, Silva AM, Singer D, Smenes BT, Soares FAA, Sobotka O, Sokolova I, Spinazzi M, Stankova P, Stier A, Stocker R, Sumbalova Z, Suravajhala P, Swerdlow RH, Swiniuch D, Szabo I, Tanaka M, Tandler B, Tavernarakis N, Tepp K, Thyfault JP, Tomar D, Towheed A, Tretter L, Trifunovic A, Trivigno C, Tronstad KJ, Trougakos IP, Tyrrell DJ, Urban T, Valentine JM, Velika B, Vendelin M, Vercesi AE, Victor VM, Villena JA, Vitorino RMP, Vogt S, Volani C, Votion DM, Vujacic-Mirski K, Wagner BA, Ward ML, Watala C, Wei YH, Wieckowski MR, Williams C, Wohlwend M, Wolff J, Wuest RCI, Zaugg K, Zaugg M, Zischka H, Zorzano A, Supporting:, Bernardi P, Boetker HE, Bouitbir J, Coker RH, Dubouchaud H, Dyrstad SE, Engin AB, Gan Z, Garlid KD, Haendeler J, Hand SC, Hepple RT, Hickey AJ, Hoel F, Kainulainen H, Kowaltowski AJ, Lane N, Lenaz G, Liu SS, Mazat JP, Methner A, Nedergaard J, Pallotta ML, Parajuli N, Pettersen IKN, Porter C, Sazanov LA, Skolik R, Sonkar VK, Vieyra A (2018) MitoEAGLE preprint
Abstract: As the knowledge base and importance of mitochondrial physiology to human health expands, the necessity for harmonizing nomenclature concerning mitochondrial respiratory states and rates has become increasingly apparent. Clarity of concept and consistency of nomenclature are key trademarks of a research field. These features facilitate effective transdisciplinary communication, education, and ultimately further discovery. Peter Mitchell’s chemiosmotic theory establishes the mechanism of energy transformation and coupling in oxidative phosphorylation. The unifying concept of the protonmotive force provides the framework for developing a consistent theoretical foundation of mitochondrial physiology and bioenergetics. We follow IUPAC guidelines on terminology in physical chemistry, extended by considerations on open systems and irreversible thermodynamics. The concept-driven constructive terminology incorporates the meaning of each quantity and aligns concepts and symbols to the nomenclature of classical bioenergetics. In the frame of COST Action MitoEAGLE open to global bottom-up input, we endeavour to provide a balanced view on mitochondrial respiratory control and a critical discussion on reporting data of mitochondrial respiration in terms of metabolic flows and fluxes. Uniform standards for evaluation of respiratory states and rates will ultimately support the development of databases of mitochondrial respiratory function in species, tissues, and cells. • Keywords: Mitochondrial respiratory control, coupling control, mitochondrial preparations, protonmotive force, uncoupling, oxidative phosphorylation, OXPHOS, efficiency, electron transfer, ET; proton leak, LEAK, residual oxygen consumption, ROX, State 2, State 3, State 4, normalization, flow, flux, O2 • Bioblast editor: Gnaiger E
Executive summary
Updated 2018-03-03
- In view of broad implications on health care, mitochondrial researchers face an increasing responsibility to disseminate their fundamental knowledge and novel discoveries to a wide range of stakeholders and scientists beyond the group of specialists. This requires implementation of a commonly accepted terminology within the discipline and standardization in the translational context. Authors, reviewers, journal editors, and lecturers are challenged to collaborate with the aim to harmonize the nomenclature in the growing field of mitochondrial physiology and bioenergetics.
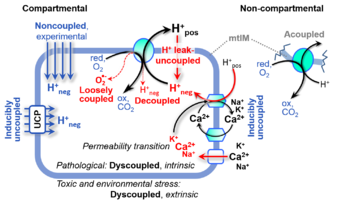
- Aerobic respiration depends on the coupling of phosphorylation (ADP → ATP) to O2 flux in catabolic reactions. Coupling in oxidative phosphorylation is mediated by translocation of protons across the inner mitochondrial membrane through proton pumps generating or utilizing the protonmotive force, measured between the mitochondrial matrix and intermembrane compartment. Compartmental coupling distinguishes vectorial oxidative phosphorylation from glycolytic fermentation as the counterpart of cellular core energy metabolism (Fig. 1).
- To exclude fermentation and other cytosolic interactions from exerting an effect on mitochondrial metabolism, the barrier function of the plasma membrane must be disrupted. Selective removal or permeabilization of the plasma membrane yields mitochondrial preparations—including isolated mitochondria, tissue and cellular preparations—with structural and functional integrity. Then extra-mitochondrial concentrations of fuel substrates, ADP, ATP, inorganic phosphate, and cations including H+ can be controlled to determine mitochondrial function under a set of conditions defined as coupling control states. A concept-driven terminology of bioenergetics incorporates in its terms and symbols explicitly information on the nature of respiratory states, that makes the technical terms readily recognized and more easy to understand.
- Mitochondrial coupling states are defined according to the control of respiratory oxygen flux by the protonmotive force. Capacities of oxidative phosphorylation and electron transfer are measured at kinetically saturating concentrations of fuel substrates, ADP and inorganic phosphate, or at optimal uncoupler concentrations, respectively. Respiratory capacity is a measure of the upper bound of the rate of respiration, depends on the substrate type undergoing oxidation, and provides reference values for the diagnosis of health and disease, and for evaluation of the effects of Evolutionary background, Age, Gender and sex, Lifestyle and Environment (EAGLE).
- Incomplete tightness of coupling, i.e., some degree of uncoupling relative to the substrate-dependent coupling stoichiometry, is a characteristic of energy-transformations across membranes. Uncoupling is caused by a variety of physiological, pathological, toxicological, pharmacological and environmental conditions that exert an influence not only on the proton leak and cation cycling, but also on proton slip within the proton pumps and the structural integrity of the mitochondria. A more loosely coupled state is induced by stimulation of mitochondrial superoxide formation and the bypass of proton pumps. In addition, uncoupling by application of protonophores represents an experimental intervention for the transition from a well-coupled to the noncoupled state of mitochondrial respiration.
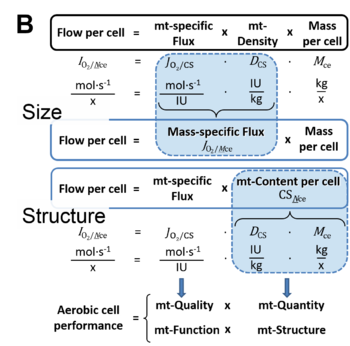
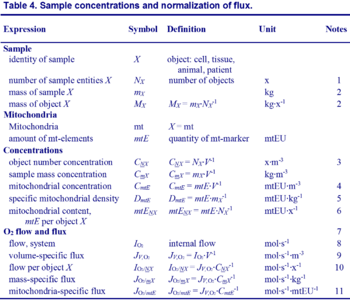
- Respiratory oxygen consumption rates have to be carefully normalized to enable meta-analytic studies beyond the specific question of a particular experiment. Therefore, all raw data should be published in a supplemental table or open access data repository. Normalization of rates for the volume of the experimental chamber (the measuring system) is distinguished from normalization for: (1) the volume or mass of the experimental sample; (2) the number of objects (cells, organisms); and (3) the concentration of mitochondrial markers in the chamber.
- The consistent use of terms and symbols discussed in this MitoEAGLE position statement will facilitate transdisciplinary communication and support further development of a database on bioenergetics and mitochondrial physiology. The present considerations are focused on studies with mitochondrial preparations. These will be extended in a series of reports on pathway control of mitochondrial respiration, the protonmotive force, respiratory states in intact cells, and harmonization of experimental procedures.
Section 2: Oxidative phosphorylation and coupling states in mitochondrial preparations
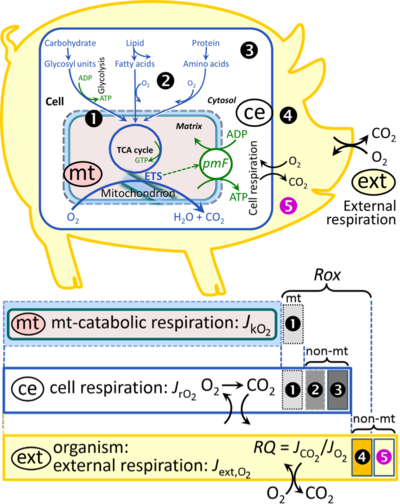
Figure 2. Cell respiration and oxidative phosphorylation (OXPHOS). Mitochondrial respiration is the utilization of fuel substrates for electron transfer to O2 as the electron acceptor. For explanation of symbols see also Figure 1. (A) Respiration in intact cells: Mitochondrial fuel substrates are the products of extra-mitochondrial catabolism of macrofuels or are taken up by the cell as small molecules. Many fuel substrates are catabolized to acetyl-CoA or glutamate, and further electron transfer reduces nicotinamide adenine dinucleotide to NADH or flavin adenine dinucleotide to FADH2. In aerobic respiration, electron transfer is coupled to the phosphorylation of ADP to ATP, with energy transformation mediated by the protonmotive force, ∆p. Anabolic reactions are linked to catabolism, both by ATP as the intermediary energy currency and by small organic precursor molecules as building blocks for biosynthesis (not shown). Glycolysis involves substrate-level phosphorylation of ADP to ATP in fermentation without utilization of O2. In contrast, extra-mitochondrial oxidation of fatty acids and amino acids proceeds partially in peroxisomes without coupling to ATP production: acyl-CoA oxidase catalyzes the oxidation of FADH2 with electron transfer to O2; amino acid oxidases oxidize flavin mononucleotide FMNH2 or FADH2. Coenzyme Q, Q, and the cytochromes b, c, and aa3 are redox systems of the mitochondrial inner membrane, mtIM. Dashed arrows indicate the connection between the redox proton pumps (respiratory Complexes CI, CIII and CIV) and the transmembrane ∆p. Mitochondrial outer membrane, mtOM; glycerol-3-phosphate, Gp; tricarboxylic acid cycle, TCA cycle.
(B) Respiration in mitochondrial preparations: The mitochondrial electron transfer system (ETS) is fuelled by diffusion and transport of substrates across the mitochondrial outer and inner membrane and consists of the matrix-ETS and membrane-ETS. ET-pathways are coupled to the phosphorylation-pathway. ET-pathways converge at the N-junction and Q-junction. Additional arrows indicate electron entry into the Q-junction through electron transferring flavoprotein, glycerophosphate dehydrogenase, dihydro-orotate dehydrogenase, choline dehydrogenase, and sulfide-ubiquinone oxidoreductase. The dotted arrow indicates the branched pathway of oxygen consumption by alternative quinol oxidase (AOX). The H+pos/O2 ratio is the outward proton flux from the matrix space to the positively (pos) charged vesicular compartment, divided by catabolic O2 flux in the NADH-pathway. The H+neg/P» ratio is the inward proton flux from the inter-membrane space to the negatively (neg) charged matrix space, divided by the flux of phosphorylation of ADP to ATP. These are not fixed stoichiometries due to ion leaks and proton slip.
(C) Phosphorylation-pathway catalyzed by the proton pump F1FO-ATPase (F-ATPase, ATP synthase), adenine nucleotide translocase, and inorganic phosphate transporter. The H+neg/P» stoichiometry is the sum of the coupling stoichiometry in the F-ATPase reaction (2.7 H+pos from the positive intermembrane space, 2.7 H+neg to the matrix, i.e., the negative compartment) and the proton balance in the translocation of ADP3-, ATP4- and Pi2-. Modified from (B) Lemieux et al 2017 and (C) Gnaiger 2014 MitoPathways.
Figure 3. Coupling in oxidative phosphorylation (OXPHOS). Modified after Gnaiger 2014 MitoPathways.
Section 3: Normalization: fluxes and flows
Concept
- Citation:
- MitoEAGLE preprint 2018-02-08(Version #). Mitochondrial respiratory states and rates: Building blocks of mitochondrial physiology Part 1. - http://www.mitoeagle.org/index.php/MitoEAGLE_preprint_2018-02-08
- Acknowledgements: We thank M. Beno for management assistance. This publication is based upon work from COST Action CA15203 MitoEAGLE, supported by COST (European Cooperation in Science and Technology), and K-Regio project MitoFit (E.G.).
- To co-authors, editors, reviewers, and readers
- The global MitoEAGLE network made it possible to collaborate with more than 250 co-authors to reach consensus on the present manuscript. Nevertheless, we do not consider scientific progress to be supported by ‘declaration’ statements (other than on ethical or political issues). Our manuscript aims at providing arguments for further debate rather than pushing opinions. We hope to initiate a much broader process of discussion and want to raise the awareness on the importance of a consistent terminology for the reporting of scientific data in the field of bioenergetics, mitochondrial physiology and pathology. Quality of research requires quality of communication. Some established researchers in the field may not want to re-consider the use of jargon which has become established despite deficiencies of accuracy and meaning. In the long run, superior standards will become accepted. We hope to contribute to this evolutionary process, with an emphasis on harmonization rather than standardization.
- To co-authors, editors, reviewers, and readers
Authors
- This is an open invitation to scientists and students to join as co-authors, to provide a balanced view on mitochondrial respiratory control and a consensus statement on reporting data of mitochondrial respiration in terms of metabolic flows and fluxes.
- Co-authors: Confirming to have read the final manuscript, possibly to have made additions or suggestions for improvement, and to agree to implement the recommendations into future manuscripts, presentations and teaching materials. (alphabetical)
- Supporting: Confirming to have read the final manuscript, and to agree to implement the recommendations into future manuscripts, presentations and teaching materials; not included in journal-publication.
- We continue to invite comments and suggestions, particularly if you are an early career investigator adding an open future-oriented perspective, or an established scientist providing a balanced historical basis. Your critical input into the quality of the manuscript will be most welcome, improving our aims to be educational, general, consensus-oriented, and practically helpful for students working in mitochondrial respiratory physiology.
- To join as a co-author, please feel free to focus on a particular section, providing direct input and references, and contributing to the scope of the manuscript from the perspective of your expertise. Your comments will be largely posted on the discussion page of the MitoEAGLE preprint website.
- If you prefer to submit comments in the format of a referee's evaluation rather than a contribution as a co-author, we will be glad to distribute your views to the updated list of co-authors for a balanced response. We would ask for your consent on this open bottom-up policy.
Progress
- Phase 1: 44 versions until 2017-09-18 / Discussion
- This manuscript on ‘Mitochondrial respiratory states and rates’ is a position statement in the frame of COST Action CA15203 MitoEAGLE. The list of co-authors evolved beyond phase 1 in the bottom-up spirit of COST.
- Phase 2: 2017-09-21 21 versions until 2018-02-06 ‘The protonmotive force and respiratory control’ / Discussion
- 2017-09-21 -: MitoEAGLE preprint - with updates on route to let the final publication fly.
- 2017-11-11: Print version (16) for MiP2017 and MitoEAGLE workshop in Hradec Kralove
- MiP2017/MitoEAGLE Hradec Kralove CZ - Discussion of manuscript submission for journal publication.
- Phase 3: 2018-02-08 (Version 22 - 23) Feedback, suggestions, and confirmation from co-authors. Contact the editor(s) of our finally targeted journal(s), to obtain a first opinion if submission to this journal will be adequate. Cell Metabolism and BBA have been mentioned as possible preferences. / Discussion
- Phase 4: 2018-02-15 (Version 24 - ) Preparation of manuscript for submission to a preprint server, such as BioRxiv, and submission to CELL METABOLISM, aiming at indexing by The Web of Science and PubMed.
- We plan a series of follow-up reports by the expanding MitoEAGLE Network, to increase the scope of harmonization and facilitate global communication and collaboration. Further discussions: MitoEAGLE events, various conferences (EBEC 2018 in Budapest).
Letter to the Editors of scientific journals
- Dear Editors:
- We would like to ask you for your opinion about the increasingly urgent issue of nomenclature in mitochondrial physiology.
- With your collaboration our goal is to make publications with data on mitochondrial respiration more generally comprehensible and data more universally useful by bringing better standardization of nomenclature and data presentation to the field.
- As the knowledge base and importance of mitochondrial physiology to human health expand, the necessity for harmonizing nomenclature concerning mitochondrial respiratory states and rates has become increasingly apparent. In the frame of COST Action MitoEAGLE, we endeavour to provide a balanced view on mitochondrial respiratory control and a critical discussion on reporting data of mitochondrial respiration. Uniform standards for evaluation of respiratory states and rates will ultimately support the development of databases of mitochondrial respiratory function in species, tissues, and cells.
- A Working Group of the COST Action MitoEAGLE is preparing a document on ‘Mitochondrial respiratory states and rates’. The group is working on it with Open Access as a ‘MitoEAGLE preprint’ and the ultimate aim of publication in a scientific journal:
- pdf preprint: » http://www.mitoeagle.org/index.php/MitoEAGLE_preprint_2018-02-08
- Executive summary: » http://www.mitoeagle.org/index.php/MitoEAGLE_preprint_2018-02-08#Executive_summary
- A Working Group of the COST Action MitoEAGLE is preparing a document on ‘Mitochondrial respiratory states and rates’. The group is working on it with Open Access as a ‘MitoEAGLE preprint’ and the ultimate aim of publication in a scientific journal:
- We would like to include your opinion as editor. We aim at providing a list of journals, from whom we received valuable feedback:
- Do you recognize a general need for a consensus on nomenclature and standards of reporting in the field of mitochondrial respiratory physiology?
- Can you provide comments and suggestions for the MitoEAGLE preprint: 'Mitochondrial respiratory states and rates’ from your point of view as an editor?
- Which further steps do you suggest towards implementing a harmonized terminology on mitochondrial states and rates in your editorial policy?
- We would like to include your opinion as editor. We aim at providing a list of journals, from whom we received valuable feedback:
- I thank you for your considerations.
- With kind regards,
- Erich Gnaiger, Ao.Univ.-Prof., Ph.D.
- Chair COST Action CA15203 MitoEAGLE - http://www.mitoeagle.org
- Chair Mitochondrial Physiology Society - http://www.mitophysiology.org
- Medical University of Innsbruck
- Department of Visceral, Transplant and Thoracic Surgery
- D. Swarovski Research Laboratory
- A-6020 Innsbruck, Austria
- Email: [email protected]
Answers
- Dear Prof. Gnaiger
- it is with great interest that I read your email. Indeed a more harmonised and well explained terminology in mitochondrial research would be excellent. I, as Editor in chief of Chemico-Biological Interactions, would support such a open source publication.
- If we at CBI can support you, e.g. in publishing this terminology review paper as an open source publication in CBI, please do contact me directly.
- Sincerely, Prof. Dr. Daniel Dietrich, Ph.D., FATS, DGPT, ERT Editor-in-Chief Chemico-Biological Interactions http://ees.elsevier.com/chembioint
- Dear Dr. Gnaiger,
- Thank you for forwarding us a copy of your manuscript under preparation. Since that the editors have already invited a submission, we recommend you submit the full paper via Editorial Manager for in-depth editorial evaluation when you are ready.
- Thank you for your support of Cell Metabolism.
- Best, Jennifer Estrompa - Journal Associate Cell Metabolism, Structure, and Cell Chemical Biology - Cell Press
- Dear Dr. Sharma,
- Thank you for asking us to consider your paper, “Mitochondrial respiratory states and rates: Building blocks of mitochondrial physiology”, for Cell Metabolism. In principle, we find the topic to be relevant and the work to be of interest. It is, however, somewhat difficult for us, based on the information you sent, to determine whether the paper would be a strong candidate for Cell Metabolism. I would therefore recommend you send us the full paper to be evaluated as part of our in-house editorial review system.
- Before submission of the article online at our EM site, please make sure that the article conforms to the format guidelines for the journal. Please mention this correspondence in the cover letter.
- We look forward to reading the full paper.
- Sincerely, Nikla Emambokus, Ph.D. - Editor-in-Chief, Cell Metabolism
- Discussion see 2018-02-15 Sharma P
- Dear Dr. Sokolova
- I have given this some thought and, after some delay for which I apologize, would like to offer a response. It is a personal one that in no way represents the views of editors of the JEB.
- I can understand why some would wish to standardize nomenclature in mitochondrial physiology. Over the years, I have come across submissions and published papers that make claims about rates measured in "state 4" when the conditions actually did not fit the definition of state 4 established decades ago and widely accepted by mitochondrial biochemists.
- However, there are terms in the list provided that are not generally accepted and I doubt whether these should be 'made standard by official declaration'. Adopting standard nomenclature would mean that deviation from it would be frowned upon by reviewers and editors. I would be more comfortable with the idea of having authors refer to (cite) your document should they wish to use any or all the terms in it. ::::: If, over time, people in the field choose to use the terms and cite the document, then the terms become accepted by consensus, rather than by declaration. This would be consistent with our wish for clarity and precision in the use of language in papers concerning mitochondrial physiology and biochemistry.
- I hope this feedback shall be of some use.
- With best wishes, Raul - Editor 'Journal of Experimental Biology'
Labels: MiParea: Respiration, mt-Awareness
Preparation: Permeabilized cells, Permeabilized tissue, Homogenate, Isolated mitochondria
Enzyme: Marker enzyme
Regulation: Coupling efficiency;uncoupling, Flux control, mt-Membrane potential, Uncoupler
Coupling state: LEAK, OXPHOS, ET
Pathway: ROX