Semantic search
| Term | Abbreviation | Description |
|---|---|---|
| Inorganic phosphate | Pi | Inorgnic phosphate (Pi) is a salt of phosphoric acid. In solution near physiological pH, the species HPO42- and H2PO4- dominate. See also: Phosphate carrier (Pic). |
| Isocitrate | isocitrate, C6H5O7-3, is a tricarboxylic acid trianion, intermediate of the TCA cycle, obtained by isomerization of citrate. The process is catalyzed by aconitase, forming the enzyme-bound intermediate cis-aconitate. | |
| Jmax | Jmax | Jmax is the maximum pathway flux (e.g. oxygen flux) obtained at saturating substrate concentration. Jmax is a function of metabolic state. In hyperbolic ADP or oxygen kinetics, Jmax is calculated by extrapolation of the hyperbolic function, with good agreement between the calculated and directly measured fluxes, when substrate levels are >20 times the c50 or p50. |
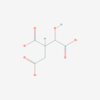
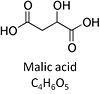
| Malate | M |
Malic acid, C4H6O5, occurs under physiological conditions as the anion malate2-, M, with pKa1 = 3.40 and pKa2 = 5.20. L-Malate is formed from fumarate in the TCA cycle in the mitochondrial matrix, where it is the substrate of malate dehydrogenase oxidized to oxaloacetate. Malate is also formed in the cytosol. It cannot permeate through the lipid bilayer of membranes and hence requires a carrier (dicarboxylate carrier, tricarboxylate carrier and 2-oxoglutarate carrier). Malate alone cannot support respiration of mt-preparations from most tissues, since oxaloacetate accumulates in the absence of pyruvate or glutamate. Malate is a type N substrate (N) required for the FAO-pathway. In the presence of anaplerotic pathways (e.g., mitochondrial malic enzyme, mtME) the capacity of the FAO-pathway can be overestimated due to a contribution of NADH-linked respiration, F(N) (see SUIT-002). |
| Malate transport | Carriers for malate: | |
| Metabolic control variable | X | A metabolic control variable X causes the transition between a background state Y (background rate YX) and a reference state Z (reference rate ZX). X may be a stimulator or activator of flux, inducing the step change from background to reference steady state (Y to Z). Alternatively, X may be an inhibitor of flux, absent in the reference state but present in the background state (step change from Z to Y). |
| Methylmalonic acid | Mma | Methylmalonic acid (Mma) is a common intermediate in many catabolic processes. In methylmalonic acidemia mitochondrial dysfunction can be observed, related to accumulation of Mma and associated with neurological symptoms. |
| MitoKit-CII | Cell permeable prodrugs, composed of MitoKit-CII/Succinate-nv and MitoKit-CII/Malonate-nv, stimulates (Snv) or inhibits (Mnanv) mitochondrial respiration in CI-deficient human blood cells, fibroblasts and heart fibres, acting on Complex II of the electron transfer system. | |
| NADH | NADH | NAD+ and NADH: see Nicotinamide adenine dinucleotide. |
| NS-pathway control state | NS, CI&II |
NS-pathway control is exerted in the NS-linked substrate state (flux in the NS-linked substrate state, NS; or Complex I&II, CI&II-linked substrate state). NS-OXPHOS capacity provides an estimate of physiologically relevant maximum mitochondrial respiratory capacity. NS is induced in mt-preparations by addition of NADH-generating substrates (N-pathway control state in combination with succinate (Succinate pathway; S). Whereas NS expresses substrate control in terms of substrate types (N and S), CI&II defines the same concept in terms of convergent electron transfer to the Q-junction (pathway control). NS is the abbreviation for the combination of NADH-linked substrates (N) and succinate (S). This physiological substrate combination is required for partial reconstitution of TCA cycle function and convergent electron-input into the Q-junction, to compensate for metabolite depletion into the incubation medium. NS in combination exerts an additive effect of convergent electron flow in most types of mitochondria. |
| Nicotinamide adenine dinucleotide | NADH | Nicotinamide adenine dinucleotide, NAD+ and NADH (pyridine nucleotide coenzymes, NAD and NADP), is an oxidation-reduction coenzyme (redox cofactor; compare FADH2). In the NADH electron transfer-pathway state fuelled by type N-substrates, mt-matrix dehydrogenases generate NADH, the substrate of Complex I (CI). The reduced N-substrate RH2 is oxidized and NAD+ is reduced to NADH,:::: RH2 + NAD+ → NADH + H+ + RThe mt-NADH pool integrates the activity of the TCA cycle and various matrix dehydrogenases upstream of CI, and thus forms a junction or funnel of electron transfer to CI, the N-junction (compare F-junction, Q-junction). NAD+ and NADH are not permeable through the mt-inner membrane, mtIM. Therefore, an increase of mitochondrial respiration after the addition of NADH may indicate an alteration of the mtIM integrity. Cytosolic NADH is effectively made available for mitochondrial respiration through the malate-aspartate shuttle or glycerophosphate dehydrogenase Complex. |
| Octanoate | Oca | Octanoate (octanoic acid). C8H16O2 Common name: Caprylic acid. |
| Octanoylcarnitine | Oct | Octanoylcarnitine is a medium-chain fatty acid (octanoic acid: eight-carbon saturated fatty acid) covalently linked to carnitine, frequently applied as a substrate for fatty acid oxidation (FAO) in mitochondrial preparations. |
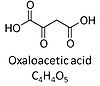
| Oxaloacetate | Oa |
Oxaloacetic acid, C4H4O5, occurs under physiological conditions as the anion oxaloacetate2-, Oa. Oxaloacetate is formed from malate by MDH. Oa reacts with acetyl-CoA through citrate synthase to form citrate, or with glutamate through transaminase to form oxoglutarate and aspartate. Oa transport is restricted across the inner mt-membrane of various tissues. Oa is a potent inhibitor of succinate dehydrogenase. |
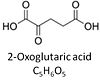
| Oxoglutarate | Og |
2-Oxoglutaric acid or alpha-ketoglutaric acid, C5H6O5, occurs under physiological conditions as the anion 2-Oxoglutarate2-, Og. 2-Oxoglutarate (alpha-ketoglutarate) is formed from isocitrate as a product of isocitrate dehydrogenase (IDH) in the TCA cycle, and is a substrate of oxoglutarate dehydrogenase (OgDH). The 2-oxoglutarate carrier exchanges malate2- for 2-oxoglutarate2- as part of the malate-aspartate shuttle. In the cytosol, oxoglutarate+aspartate are transaminated to form oxaloacetate+glutamate. Cytosolic malate dehydrogenase converts oxaloacetate+NADH to malate. |
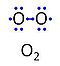
| Oxygen | O2 |
Molecular oxygen, O2 or dioxygen, has two atoms of oxygen, O, which is the chemical element with atomic number 8. The relative molecular mass of O2, Mr,O2, is 32 (or 31.9988). The element O has 8 protons, 8 neutrons and 8 electrons. In the figure, the two electrons in the first electron shell are not shown. Of the six electrons in the outer shell (blue bullets), one electron from each of the two atoms is shared in O2 forming the covalent bond, and one electron in each atom is unpaired. |
| P50 | p50 | p50 is the oxygen partial pressure at which (a) respiratory flux is 50% of maximum oxygen flux, Jmax, at saturating oxygen levels. The oxygen affinity is indirectly proportional to the p50. The p50 depends on metabolic state and rate. (b) p50 is the oxygen partial pressure at which oxygen binding (on myoglobin, haemoglobin) is 50%, or desaturation is 50%. |
| Palmitate | Paa | Palmitate is a term for the salts and esters of palmitic acid (CH3(CH2)14COOH). Palmitic acid is the first fatty acid produced during fatty acid synthesis and the precursor to longer fatty acids. Palmitate negatively feeds back on acetyl-CoA carboxylase (ACC), which is responsible for converting acetyl-CoA to malonyl-CoA, which in turn is used to add to the growing acyl chain, thus preventing further palmitate generation. In order to dissolve the water-insoluble sodium palmitate, BSA is needed to form the water-soluble compound called palmitate:BSA. |
| Palmitoyl-CoA | Pa-CoA | Palmitoyl-CoA is a coenzyme A derivative of palmitate formed by acyl-CoA synthase. In contrast to medium- and short-chain acyl-CoA, palmitoyl-CoA cannot freely diffuse into the mitochondrial matrix. Formation of palmitoylcarnitine by CPTI is necessary prior to transfer into mitochondria for further fatty acid oxidation (β-oxidation). To study Fatty acid oxidation using Palmitoyl-CoA, Carnitine and low amount of malate is needed on mitochondrial preparations. |
| Palmitoylcarnitine | Pal | Palmitoylcarnitine is an ester derivative of carnitine (long-chain acylcarnitine) involved in the metabolism of fatty acids. Within the cell, palmitoylcarnitine is transported into the mitochondria to deliver palmitate for fatty acid oxidation and energy production. |
| Phosphate | Pi | See: Inorganic phosphate |
| Phosphocreatine | PCr | Phosphocreatine is a high energy compound in the skeletal muscle of vertebrates and is present in 4 to 5 times the concentration of ATP. |
| Preparation of SUIT chemicals | Preparation of SUIT chemicals describes the preparation of chemicals used in Substrate-Uncoupler-Inhibitor Ttitration (SUIT) protocols. | |
| Product | A product in a chemical reaction has a positive stoichiometric number since it is produced, whereas a substrate has a negative stoichiometric number since it is consumed. | |
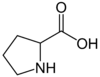
| Proline | Pro |
Proline (Pro), C5H9NO2, is an amino acid which occurs under physiological conditions mainly in the nonpolar form, with pKa1 = 1.99 pKa2 = 10.96. Proline is an anaplerotic substrate that supports both the proline pathway control state and the glutamate-anaplerotic pathway control state. Proline is used as a single substrate or in combination with carbohydrate-derived metabolites in mitochondria particularly of flight muscle of many (but not all) insects. Proline is oxidized to delta-1-pyrroline-5-carboxylate by the mtIM L-proline:quinone oxidoreductase (proline dehydrogenase, ProDH), with reduction of FAD to FADH2 and direct entry into the Q-junction. delta-1-pyrroline-5-carboxylate is converted to glutamate by 1-pyrroline-5-carboxylate dehydrogenase. |
| Prosthetic group | A prosthetic group is a cofactor that is attached permanently and tightly or even covalently to an enzyme and that is regenerated in each enzymatic turnover. Thus a prostethic group is distinguished from a coenzyme or cosubstrate that is attached loosely and transiently. Like a coenzyme, the prosthetic group is required by an enzyme for its activity. A prosthetic group is 'a tightly bound, specific nonpolypeptide unit in a protein determining and involved in its biological activity' (IUPAC definition). FMN/FMNH2 and FAD/FADH2 are prosthetic groups of Complex I and Complex II, respectively. | |
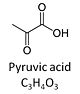
| Pyruvate | P |
Pyruvic acid, C3H4O3, is an alpha-keto monocarboxylic acid which occurs under physiological conditions mainly as the anion pyruvate-, P, with pKa = 2.5. Pyruvate is formed in glycolysis from phosphoenolpyruvate. In the cytosol, pyruvate is a substrate of lactate dehydrogenase. Pyruvate enters the mitochondrial matrix via a specific low Km' H+/monocarboxylate cotransporter known as the pyruvate carrier. Similarly, the plasma membrane of many cell types has H+/monocarboxylate cotransporter activity and pyruvate can thus be added as a substrate to living cells. In the mt-matrix the oxidative decarboxylation of pyruvate is catalyzed by pyruvate dehydrogenase and yields acetyl-CoA. Pyruvate competitively reverses the inhibition of cytochrome c oxidase by cyanide. Pyruvate is an antioxidant reacting with hydrogen peroxide. |
| Q-pools | Q | Different Q-pools are more or less clearly distinguished in the cell, related to a variety of models describing degress of Q-pool behavior. (1) CoQ-pools are distinguished according to their compartmentation in the cell: mitochondrial CoQ (mtCoQ) and CoQ in other organelles versus plasma-membrane CoQ. (2) The total mitochondrial CoQ-pool mtCoQ is partitioned into an ETS-reactive Q-pool, Qra, and an inactive mtCoQ-pool, Qia. (2a) The Qra-pool is fully reduced in the form of quinol QH2 under anoxia, and fully oxidized in the form of quinone in aerobic mitochondrial preparations incubated without CHNO-fuel substrates. Intermediate redox states of Qra are sensitive to pathway control and coupling control of mitochondrial electron transfer and OXPHOS. (2b) The Qia-pool remains partially reduced and oxidized independent of aerobic-anoxic transitions. The redox state of Qia is insensitive to changes in mitochondrial respiratory states. (3) The Qra-pool is partitioned into Q with Q-pool behavior according to the fluid-state model (synonymous: random-collision model) and Q tightly bound to supercomplexes according to the solid-state model. The two models describe the extremes in a continuum of homogenous or heterogenous Q-pool behavior. The CII-Q-CIII segment of the S-pathway is frequently considered to follow homogenous Q-pool behavior participating in the Qhom-pool, whereas the CI-Q-CIII segment of the N-pathway indicates supercomplex organization and metabolic channeling with different degrees of Q-pool heterogeneity contributing to the Qhet-pool. |
| Reactive nitrogen species | RNS | Reactive nitrogen species, RNS, are nitric oxide-derived oxidants. The main source of RNS is nitric oxide (NO•). NO• plays an important role in cell signaling and in oxidative-nitrosative stress. |
| Reactive oxygen species | ROS | Reactive oxygen species, ROS, are molecules derived from molecular oxygen, including free oxygen radicals, which are more reactive than O2. Physiologically and pathologically important ROS include superoxide, the hydroxyl radical and hydroxide ion, hydrogen peroxide and other peroxides. These are important in cell signalling, oxidative defence mechanisms and oxidative stress. |
| Substrate | IUPAC distinguishes three definitions of 'substrate': (1) The chemical entity whose conversion to a product or products is catalysed by one or several enzymes. (2) A solution or dry mixture containing all ingredients which are necessary for the growth of a microbial culture or for product formation. (3) Component in the nutrient medium, supplying the organisms with carbon (C-substrate), nitrogen (N-substrate), etc. A substrate in a chemical reaction has a negative stoichiometric number since it is consumed, whereas a product has a positive stoichiometric number since it is produced. | |
| Substrate-uncoupler-inhibitor titration | SUIT | Mitochondrial Substrate-uncoupler-inhibitor titration (SUIT) protocols are used with mitochondrial preparations to study respiratory control in a sequence of coupling and substrates states induced by multiple titrations within a single experimental assay. |
| Substrates as electron donors | Sred | Substrates as electron donors are reduced fuel compounds Sred that are oxidized to an oxidized product Pox during H+-linked electron transfer, Sred → Pox + 2{H+ + e-}. Mitochondrial respiration depends on a continuous flow of electron-supplying substrates across the mitochondrial membranes into the matrix space. Many substrates are strong anions that cannot permeate lipid membranes and hence require carriers. |
| Succinate | S |
Succinic acid, C4H6O4, (butanedioic acid) is a dicarboxylic acid which occurs under physiological conditions as the anion succinate2-, S, with pKa1 = 4.2 and pKa2 = 5.6. Succinate is formed in the TCA cycle, and is a substrate of CII, reacting to fumarate and feeding electrons into the Q-junction. Succinate (CII-linked) and NADH (CI-linked) provide convergent electron entries into the Q-junction. Succinate is transported across the inner mt-membrane by the dicarboxylate carrier. The plasma membrane of many cell types is impermeable for succinate (but see Zhunussova 2015 Am J Cancer Res for an exception). Incubation of mt-preparations by succinate alone may lead to accumulation of oxaloacetate, which is a potent inhibitor of Complex II (compare Succinate and rotenone). High activities of mt-Malic enzyme (mtME) prevent accumulation of oxaloacetate in incubations with succinate without rotenone. |
| Succinate transport | The dicarboxylate carrier catalyses the electroneutral exchange of succinate2- for HPO4-2-. | |
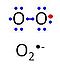
| Superoxide | O2•- |
Superoxide anion, O2•-, is a free radical formed in a one-electron reduction of molecular oxygen (red bullet in the figure), yielding a negatively charged molecule with a single unpaired electron (blue bullet on the left). It is highly reactive with organic compounds, and its intracellular concentration is kept under control by superoxide dismutase. |
| TMPD | Tm | N,N,N',N'-Tetramethyl-p-phenylenediamine dihydrochloride, TMPD, is applied as an artificial substrate for reducing cytochrome c in the respirometric assay for cytochrome c oxidase (CIV) activity. It is maintained in a reduced state by ascorbate and undergoes autoxidation as a function of oxygen pressure, TMPD, ascorbate and cytochrome c concentration. |
| Taurine | Taurine, or 2-Aminoethan sulfonic acid, is one of the most abundant low-molecular-weight organic constituents in animals and humans. It has a multitude of functions in different types of tissue, one of which is the stabilization of membranes. Because of this and its antioxidative effect, taurine is a component of the respiration media MiR05 and MiR06 to preserve mitochondrial function. | |
| Tetrahydrofolate | THF | Tetrahydrofolate, THF, is the substrate in mitochondrial folate-mediated 1C metabolism, an NADH-linked pathway leading to the formation of formate which is exported to the cytosol. |
| Volume of the solute | Most of the chemicals for SUIT protocol titrations are prepared by weighing the substance on the balance, transferring to a volumetric glass flask and adding solvent until the intended volume is reached. However, for practical reasons some of the chemical compounds are prepared by just adding the solvent instead of adjusting it's volume. For example, this approach is useful if the substance is very toxic. Then an arbitratry amount is taken, its mass determined on the balance without trying to reach a specific value and the necessary amount of solvent is added. Adding the solvent instead of adjusting its volume is also useful if small amounts are needed (e.g. 1 mL) or if the compound has to be prepared directly before using it like Pyruvate. In these cases the volume contributed by the solute was tested. | |
| Water | H2O |
Water, H2O, is widely used in the laboratory, particularly as a solvent and cleaning agent. Chemically pure water is prepared in various grades of purification: double distilled water (ddH2O) versus distilled water (dH2O or aqua destillata, a.d.) and deionized or demineralized water (diH2O) with various combination purification methods. When H2O is mentioned without further specification in published protocols, it is frequently assumed that the standards of each laboratory are applied as to the quality of purified water. Purification is not only to be controlled with respect to salt content and corresponding electrical conductivity (ultra-pure water: 5.5 μS/m due to H+ and OH- ions), but also in terms of microbial contamination. |